Stepanenko T., Bashevaya T.
Donbas National Academy of Civil Engineering and Architecture,
Ukraine
EMISSION REDUCTION OF POLLUTANTS IN
THE PETROCHEMICAL
INDUSTRY
Chemical and petrochemical industries are the sources of multicomponent
emissions of chemical impurities I, II, III, IV hazard class (organized process
emissions, ventilation emissions, open areas with equipment) into the
environment.
Work objective: to explore and
study the method of emission reduction of repugnant substances in the
production of phenol and acetone.
In the
production of phenol and acetone, by the cumene method, which is widely used in
the present time, we can distinguish three main stages: alkylation of benzene
with propylene to form isopropylbenzene (the reaction proceeds at a temperature
of 250°C and a pressure of 2.5 MPa in the presence of a catalyst); the
oxidation of cumene by atmospheric oxygen in hydroperoxide at 100-130°C; the degradation
of cumene hydroperoxide to phenol and acetone at 50-90°C [1]. At that, the degradation
products of cumene hydroperoxide, passing to the atmosphere, are formed such as
carbon dioxide, methane, ethylene, acetophenone, biphenyl, phenol, α-methylstyrene.

There
are two ways to reduce the amount of pollutants released into the environment
·
cleaning of gas-air mixture;
·
prevention of pollution by optimizing the existing process flows or
introduction of new unwaste technologies.
The analysis
of the literature showed that to reduce emissions of the mentioned substances,
we could use different methods. Absorption technique is widely used [2].
However, a significant weakness is the formation of liquid flows to be
recycled. Sufficiently widely spread got the adsorption technique of pollution
abatement [2]. A large specific consumption of the desorption stages and
subsequent separation greatly complicates the application of this method for
multicomponent mixtures. The advantage of catalytic and catalytic thermal
methods is the high degree of purification (95-99%), ability to process
multi-component gases with low initial emission concentrations. Along with
this, these methods have a drawback - the high cost of catalysts [2].
An
alternative way to reduce harmful emissions into the atmosphere is to optimize
the technological process of production of phenol and acetone.
When
coupled with the production of phenol and acetone by the cumene stage of cumene
oxidation by air oxygen simultaneously with cumene hydroperoxide, various
by-products are formed, such as: dimethylphenylcarbinol peroxide of dicumene, acetophenone,
α-methylstyrene, as well as
phenol and organic acids, which require disposal. The presence of these
components is considered harmful for the reaction. Indeed, acid favor the
splitting of hydroperoxide into phenol, which itself is an inhibitor of the
oxidation reaction [3].
It should be noted that the most toxic substances are phenols (MAC =
0,003mg/m3), acetophenone (MAC = 0,003 mg/m3), α-methylstyrene (MAC = 0,04mg/m3).
Effect of the temperature on the
composition of the breakdown products of cumene hydroperoxide is shown in
Figure 1.
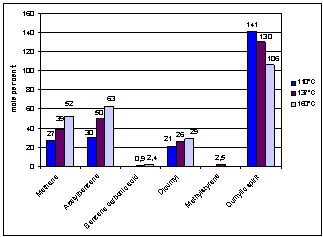
Fig. 1 – Composition of the breakdown products of cumene hydroperoxide at
110°C, 137°C and 160°C
It is
known from the literary sources, that the on-process temperature affects the
amount of breakdown products of cumene hydroperoxide, which enters the
atmosphere (Fig. 1). The figure shows that fall of the temperature of the on-process
at 50ºC (from 160ºC until 110ºC) allows a factor of two to
reduce the methane content and acetophenon in flue gases. We also know that at
60°C, the decomposition reaction of cumene hydroperoxide does not proceed.
Adding reagent, - the initiator of oxidation, - solves this problem and lets to
reduce the oxidation temperature to 35°C [5].
We have
investigated the rate of oxidation of cumene hydroperoxide (in the presence of
an oxidation initiator - N-hydroxyphthalimide) at different temperatures. The
introduction of the initiators of the process lets to reduce the on-process
temperature to 35°C [5]. The temperature
range was chosen as 35 - 60°C.
To
calculate the rate of oxidation of cumene hydroperoxide, we used the data from
the volume of absorbed oxygen, obtained by the experimental means. Dependence
of the rate of cumene oxidation on the temperature in the presence of an
initiator is shown in Fig. 2.
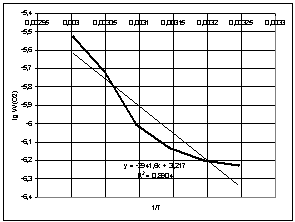
Fig. 2 – A plot of oxidation rate of cumene hydroperoxide on the
temperature
Dependence of
the rate of oxidation in the temperature range (35-60°C) is given by: ![]() . With a decrease of temperature process in 5°C, the oxidation rate falls by 1,5
times, while reducing by 10°C – 3 times. Analyzing the experimentally derived
data, we can conclude that the maximum oxidation rate of cumene in the presence
of oxidation initiator (N- hydroxyphthalimide) was observed at 60°C (333 K).
. With a decrease of temperature process in 5°C, the oxidation rate falls by 1,5
times, while reducing by 10°C – 3 times. Analyzing the experimentally derived
data, we can conclude that the maximum oxidation rate of cumene in the presence
of oxidation initiator (N- hydroxyphthalimide) was observed at 60°C (333 K).
The comparative characteristics of the breakdown
products of cumene hydroperoxide, which enters the atmosphere at a temperature
of 60°C and 110°C is shown below (in mol.%):
|
|
60 °С |
110 °С |
|
Methane |
12 |
27 |
|
Acetyl
benzene |
6 |
30 |
|
Dicumyl |
11 |
21 |
|
α-methylstyrene |
0,35 |
0,7 |
Reducing of harmful emissions during production of phenol and acetone is
achieved by introducing the catalyst N-hydroxyphthalimide, that lets to reduce
the temperature from 110°C till 60°C. The decrease in temperature of oxidation
process of cumene in 2 times allows to reduce the number of pollutants,
released into the atmosphere: acetophenone – 5 times; Dicumyl – 1,9 times;
methane – 2,25 times, α-methylstyrene – 2
times.
References:
1.
Кружалов Б.Д. Совместное
получение фенола и ацетона. / Б.Д. Кружалов, Б.Н.Голованенко. – M.: Химия, 1983. – 200 с.
2.
Кутепов А.М. Общая
химическая технология. / А.М. Кутепов, Т.И. Бондарева, М.Г. Беренгартен. – М.:
Высшая школа, 1990. – 512 с.
3.
Пат. 2131414 Российская Федерация, МПК7
С 07 С 409/10 С 07 С 407/00. Способ получения гидропероксида
кумола / Рон-Пуленк
Шими;
заявитель и патентообладатель. – № 96105025/04; заявл. 08.08.1994; опубл. 10.06.1999.
4.
Андреас Ф. Химия и
технология пропилена. / Ф. Андреас, К.
Гребе – Л.: Химия, 1973. – 368 с.
5.
Горбатенко Н.В. Дія
супрамолекулярних галоїдвмісних комплексів на розпад ацилпероксидів та
ініціювання ними радикально-ланцюгового окиснення: дис.
... канд. наук: 02.00.04 / Горбатенко Наталія
Валеріївна. – Донецьк, 2006. – 144с.