Stud . Doroshko Yu. M., stud.
Borodina O.O., c.b.s. Lych I.V., stud. Shulzhenko V. S.
National University of
Food Technologies
BIOENGINEERING STRUCTURES BASED ON ABZYMES
Medical
abzymology achievements became the basis for developing new drugs. Catalytic antibodies
(Ab) are defined as "new molecular tool" to explore rheumatologic and
cardiac diseases, diseases of endocrine system, autoimmune diseases of the
central nervous system (CNS), sepsis, HIV and other infectious and neoplastic
lesions [1].
Beside
various aspects of abzymes direct clinical usage, a particular interest to
researchers, experts and healthcare provider causes an abzyme-based
bioengineered structures with their further usage in fundamental and practical
medicine.
There
are two types of abzymes we know: natural and artificial.
Artificial
abzymes - is hydrolyzed esters of dinitrophenol (DNP). During many diseases
(autoimmune, viral and oncological) in human body are produced antibodies that
hydrolyze peptides, proteins, DNA, RNA and polysaccharides. Such catalytically
active antibodies called ‘natural’ [1].
We
know that women hormonal and immune condition changes related to pregnancy,
childbirth and lactation and may lead to autoimmune diseases development. Because
of the autoimmune tolerance violation in women body appear highly specific
auto-Ab and abzymes, determination of which may be useful for predicting the
emergence of autoimmune diseases.
Catalytic
Ab with therapeutic functions that will be further used in modern treatment
regimens must meet two main criteria - to recognize and bind to a cell (or target
molecule) and after specifically and selectively linked them, take adequate
catalytic functions.
In
addition, there are active development process in a use of DNA-abzymes as
molecular probes and diagnostic tools to treat patients with fungal airway
disease [2]. It is possible that in the catalytic activity of auto-Ab (DNA abzymes
for particular) are programmed an additional enzymatic Rh, implementation of
which is related to a specific conditions of metabolism, such as the formation
of autoimmune conflict, against infection or during pregnancy [2].
Another
characteristic feature of abzymes allows to turn singlet oxygen into hydrogen
peroxide. Researchers believe that immunoglobulins – a unique class of proteins
that are capable of generating up to 500 molar equivalents of hydrogen peroxide
from singlet oxygen without activity reduction due redox process of molecule that
play catalyst role. It should be noted, that other proteins have ten times less
activity; moreover, they are rapidly inactivates during redox reactions [3].
Next
direction of abzymes usage, most dynamically developing one to date, associated
with the creation of catalytic Ab, that are capable to bind and destroy narcotic
drugs, circulating in the peripheral blood until the initialization their toxic
effects on the nervous system and other systems and tissues [4].
Proved
that catalytic antibodies can effectively neutralize toxic organophosphorus
compound (toxic OPC). The resulting catalytic monoclonal Ab contribute to the
rapid hydrolysis of stable toxic OPC. It makes possible to use catalytic Ab
aiming to prevent and treat the most severe intoxication, caused by toxic OPC.
This abzymes application is very promising in our time.
With
unique catalytic and cytotoxic properties, DNA-abzymes can act as a powerful
regulator of apoptosis and other mechanisms in systemic autoimmune and cancer
diseases, while claiming the role of an additional tool in the diagnosis.
Therefore,
it is possible to say that the perspective of abzymes usage for the tumors
treatment is one of the important areas of catalytic Ab application that
develops right now. In this case, abzymes chosen as activators of cytostatic
drugs to minimize their overall toxic effect on the body [5]. Specifically, during
breast cancer disease is used a bizarre Ab, one active center of which aims to
integrate the receptor of tumor cells (concentration of those receptors in the
tumor is significantly higher), while another Ab’s active center has catalytic
function, which turns doxorubicin predecessor into its active cytostatic form
[6]. During treatment, Ab selectively accumulated in the tumor and then show
their catalytic activity. Thus, a concentration of active cytostatics rapidly
increases in the tumor without a significant increase in activity of the drug
in other organs and systems, which significantly reduces its overall toxic
effect.
Other
perspective usage of abzymes is a new generation drugs development for
site-directed anticancer chemotherapy programs, where instead of traditional
bacterial enzymes activators (ADEPT, antibody-directed enzyme prodrug therapy),
used abzymes that activates drug precursor (ADEPT) at the time of their
delivery to the tissues and target organs. The foundation of this therapy is
the use of antibodies conjugated with enzymes. This allows combining two
important functions of antibodies, such as cell recognition and catalyst. ADEPT
principle based on the specific interaction of tumor-binding antigen with the
antibody and prodrug activating process by enzyme. This allows to avoid drug’s overall
toxicity and allows to keep the reaction ongoing as closest to cancer cells as
possible. The usage of non-human enzymes in human therapy increases the risk of
immunogenicity and limits chances of potential therapeutic protein re-use. In
this case, enzymes may be replaced by abzymes.
Humanization
of antibodies by genetic engineering also allows resolving the immunogenicity issue.
Currently, in the prodrug therapy following abzymes are used: 38C2, 33F12, 84G3
and 93F3, but the most studied are 38C2. This antibody detects aldolase
activity and generated by reactive immunization.
Abzyme
38C2 activates prodrug form of such anticancer drugs as doxorubicin and camptothecin.
38C2 has the ability to inhibit a growth of primary and metastatic tumors,
including Kaposi's sarcoma, melanoma and breast cancer. Antibodies, which used
in abzyme’s prodrug therapy is an ADEPT strategy option, while abzymes that
replaces enzymes in this therapy called bispecific (Figure 1). However, the usage
of bispecific abzymes are not widely popular in clinical trials [6].
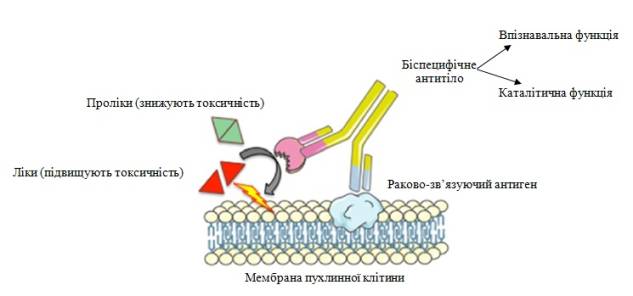
Fig.1 ADEPT strategy mechanism
New pharmacological structures, that composed of
adapted to the human body abzymes, allows to more effectively and efficiently
to apply prodrug-therapy methods in the complex treatment of a number of
malignant tumors [6].
As a result, abzymes begins to be considered as
potential ‘magic bullet’, which will allow to recognize and selectively destroy
tumor cells without damaging the healthy ones [7]. The ‘magic bullets’
mechanism is based on a composing the target cell with a cytotoxic fragment.
The combination of the target molecule with the cytotoxic molecule allows to
selectively destroy cells that express the target molecule on its surface. In
the cancer treatment as the target molecule is used the antigen, that is
selectively expressed by tumor cells or blood vessels cells, which support
tumor growth. The usage of catalytic antibodies during tumor diseases has
number of advantages compared to ordinary enzymes, since human enzymes is
limited due prodrug activation by endogenous enzymes in the blood and normal
tissue of the patient. To overcome these limitations, researchers have
suggested replacing the enzyme component with catalytic antibody.
Considering the data from latest academic publishing,
it is safe to say that the potential usage of catalytic antibodies for
selective chemotherapy convincing one, as both reaction are not catalyzed by
human enzymes; low immunogenicity option due humanizing antibodies is also
possible. Bifunctional antibody, which involved in this process, consists of
two antibodies: targeted and catalytic. Immunoglobulin G (IgG) molecule is used
as such bifunctional antibody [7]. As a result, the enzymatic component is
replaced with catalytic antibody.
Therefore, can be said, that in the nearest future
bioengineering catalytic antibodies with certain specificity and properties may
become the basis for creating drugs that can recognize tumor target tissue and
selectively destroy it, while leaving healthy cells intact.
References:
1.
Ìàëüöåâ Ê. À., Õèòðîâ À. Í., Ââåäåíñêàÿ Î. Þ. è äð. Êàòàëèòè÷åñêèå
àóòîàíòèòåëà – íîâûé ìîëåêóëÿðíûé èíñòðóìåíò â êàðäèîëîãèè è îôòàëüìîëîãèè // Òåð. àðõ. – 2006. – T. 16. ¹ 11. – Ñ. 70–76.
2.
Purkayastha
S., Madan T., Shah A., Krishnamurthy H.G. Multifunctional
antigens of A. Fumigatus and specific antibodies // Appl. Biochem. Biotechnol. – 2008. – V. 83. ¹ 1/3. – P. 297–313.
3.
Wentworth P., Jones L. H., Wentworth A. D. et
al. Antibody
catalysis of the oxidation of water // Science. – 2009.
– V. 23. ¹ 12.
– P. 1806–1811.
4.
Bosron
W.F., Hurley T.D. Lessons from a
bacterial cocaine esterase // Nat. Struct. Biol. – 2010. – V. 9.
¹ 12. – P. 4–5.
5.
Sinha S. C., Li L. S.,
Watanabe
S. et al. Aldolase antibody activation
of prodrugs of potent aldehyde containing cytotoxics for selective chemotherapy
// 2004. – V.
10. ¹ 21. –
P. 5467–
5472.
6.
Abraham S., Guo F., Li L. S. ct at. Synthesis of the
next-generation therapeutic antibodies that combine cell targeting and antibody-catalyzed
activation // Proc. Nati. Acad. Sci. USA. – 2007. – V. 104. ¹ 13 – P. 5584–5589.
7.
Severine
P. L., Raouia B. N. Catalytic antibodies and their applications in biotechnology: state of
the art // Biotechnol. Lett. – 2014. – V. 37. ¹ 20. – P. 69–81.