dr
inż. Jerzy Stanisław Kowalski ,dr inż. Marek Mazur
dr hab. inż. Wojciech
Wojciechowski
Institute
of Materials Engineering
Cracow
University of Technology
Pad welding as a means of protection
against corrosion
Abstract
The
article presents the possibilities that the arc pad welding creates in
protecting from corrosion various materials used in construction of equipment
operating in power industry. By application of pad welding, a coating (layer)
is deposited which not only protects the
material from corrosion caused by the flow of more or less aggressive media
(e.g. water, waste gases) but also prolongs the life of pad welded elements
since padding welds are usually characterised by high resistance to abrasive
wear. Pad welding is specially suitable for repair of parts damaged or
suffering standard wear and tear during operation in water installations, power
supply systems, etc.
INTRODUCTION
Failure of pipelines handling industrial water to power plants or water
from abyssal strata is in the majority of cases caused, apart from the „human”
factor, by corrosion and erosion. This is due to the fact that water
transported by pipelines contains dissolved gases (including gases that speed
up the corrosion process), mineral compounds (e.g. calcium carbonate, chlorides
of sodium, calcium, or magnesium), and organic compounds. Additionally, water
from the subterranean springs often contains the acidic sodium carbonate,
carbon dioxide, sulphates and chlorides in concentrations much higher than the
water in surface layers. Apart from the
effect of purely chemical nature, this also influences the pH value and the
electrode process that takes place at cathode spots with reactions of hydrogen
or oxygen depolarisation. The processes of erosion that take place on the
internal surfaces of steel pipes are accelerated by the water-carried particles
of undissolved quartz and argillaceous minerals which abrade the deposits
growing on the internal pipe walls. The next factor that makes the pipeline
degradation process more intense is the water flow rate. It is generally
assumed that increasing the rate of water flow usually speeds up the corrosion
process, caused by the corrosion products washed out in a strong jet of the
flowing liquid. In the latter two cases, the processes of corrosion and erosion
are observed to proceed at a rate definitely higher in these sections of the
pipeline where there are some elements of the technical infrastructure
restricting the free water flow, like reducing pipes, orifices and, particularly, elbows (bends).
An important factor that affects the service life of pipelines is the
temperature of the handled water. This refers to both pipelines supplying water
to power plants and those which serve for drawing out and handling of abyssal
and geothermal waters. In the latter case, of particular importance is the
occurrence of temperature gradients that favour the formation of corrosion
cells.
The above mentioned factors responsible for the corrosive and erosive
degradation of water pipelines mainly depend on the properties of the handled
water, and the tools that the technical supervising personnel have to control
the processes of corrosion are in this case very limited. The possibilities to
control these phenomena are definitely greater at the stage of designing and
selecting materials for the pipeline construction, specially as regards the use
of effective methods for intraoperational upgrading. Failure of pipeline is
always a serious problem in terms of both material and economy. The repair requires
large financial outlays for replacement of the damaged section and the
necessity of arresting the operation of the whole pipeline during the repair.
There are known various methods to reduce losses caused by the erosion of water pipelines. The choice of the
best method depends on detailed analysis of the pipeline operating conditions.
In most cases, the aim of all the methods applied currently is to prolong the
life of the structural elements of a pipeline using for their construction clad
plates, steel plates after thermal-chemical treatment, plates surface etched
with acids containing corrosion inhibitors, or plates coated or painted with
anticorrosive agents. The sections of the pipelines operating under the most
trying conditions of the corrosion-threatening environment are made from the
very expensive stainless steels, and in some, economically justified, cases
from the titanium-based alloys. Nevertheless, modern technology has no
efficient technical means to totally eliminate the corrosive wear and tear of
water pipelines. Therefore, the problem of current repair of these elements is
very important. Effective and relatively
cheap means of upgrading the pipeline sections damaged by corrosion and erosion
offers the technique of pad welding. The said technology is very effective in
making up for the losses of material and filling the pittings in parent metal,
due to the application of a layer of proper material selected together with the
welding electrode. Additionally, in the case of e.g. plumbing fittings
fabricated by the technique of powder metallurgy, pad welding ensures the
removal of surface porosities present in these products. Another advantage of
the pad welding process is the possibility to use this method in repair of
castings, specially at the places where, due to a long-lasting operation, the
sub-surface defects, like pores and voids, have been detected.
In the study presented here, a method of pad welding
of the damaged surfaces of the steel
plates has been proposed as a relatively simple and effective means for their
upgrading, ensuring further failure-free operation of pipeline.
All over the world, corrosion is a serious problem. It destroys
approximately 2 – 5 % of the gross
national product, is responsible for the contamination of natural environment
and creates numerous hazards to the human health and life. In 1989, in Poland, the losses due to corrosion and the
related contamination of natural environment reached 32% of overall material
losses. The effects of corrosion are present everywhere. Corrosion affects
concrete, ferroconcrete, harbour facilities, industrial devices, military
infrastructure and military equipment. As much as 80% of all financial means
assigned for the repair of bridges and viaducts are as a matter of fact
assigned for combating the effects of corrosion which attacks the industrial
facilities and distribution systems.
CORROSION
Corrosion means all these processes
during which metal or alloy used as a structural material undergoes, under the
effect of environmental factors, a transformation from the metallic state to a
chemically bounded state.
Corrosive reactions are not always harmful to the environment. The use
of numerous reactive metals (e.g. titanium, niobium, tantalum, and rare earth
metals) is possible in some environments due to the formation of a thin layer
of corrosion products that forms a barrier impeding further progress of
reaction.
Depending on the mechanism by which the corrosion process is proceeding,
two basic types of corrosion are distinguished, viz. the chemical and
electrochemical corrosion.
In aqueous solutions, the majority of
corrosion processes take place by electrochemical route, which means that
during reactions the electric charges are crossing the phase boundary. In terms
of structure, the surface of metal is not homogeneous but consists of minute
electrodes electrically shortcircuited by the metal. As long as the metal
remains dry, the current is not passing
and the corrosion does not take place. As soon, however, as it enters in
contact with water solution or with moisture contained in the humid atmosphere,
the local cells start acting and transform the metal into a product of
corrosion. The impurities present in metal enhance the corrosion, because
acting as microcells they exert a definitely stronger effect, and for this
reason, pure metals usually corrode much more slowly than metals with some
admixtures.
WELDING OF METALS vs CORROSION
The thermal processes that take place
during joining of metallic materials cause different phase transformations
which, to a smaller or greater extent, may occur within the heat affected zone.
This is specially true in the case of welding, partly also in the case of
pressure welding and soldering. The transformations change the mechanical
properties and corrosion resistance of thus produced joints.
WELDING
Depending on the welding technique,
the type of auxiliary materials and welding parameters, the welding process can
change in a different way the physico-chemical nature of metals and alloys. The
changes involve chemical reactions that proceed more quickly at higher
temperatures and precipitation of compounds, like carbides, oxides, nitrides,
sulphides, phosphorides, and intermetallic compounds. Moreover, during the
process of welding, there is a recrystallisation of metal, the growth and
deformation of crystal grains, sometimes the formation of new structural phases
which move and agglomerate, giving rise to lattice defects and internal
stresses of uni- or biaxial character, caused mainly by the shrinking power of
the welded metal.
There is always some degree of inhomogeneity between the welded joint
and parent metal. Its symptoms are differences in structure, mechanical
properties and the state of stress inside metal. The inhomogeneity may enhance
the corrosion rate, specially as regards the electrochemical and stress-induced
corrosion. To eliminate or reduce this inhomogeneity, additional thermal or
mechanical treatments are applied.
THE CORROSION OF WELDED STEEL JOINTS
In the majority of cases, the welded
joints are less resistant to corrosion than the parent metal. Normally, all
welded structures made from carbon and low-alloy steels are protected from the
atmospheric corrosion with special coatings applied by painting. Quite often,
however, it happens so that on the welded joints the varnish is spalling and
falling off, exposing the bare metal surface, which is now running the greater
risk of corrosion. To avoid spalling of paints and enamels, the welded joints
and their neighbourhood should be cleaned mechanically before painting with,
e.g. hot water, and dried.
The laboratory tests of the
corrosion resistance of the joints welded from carbon and low-alloy steels are
carried out in accordance with PN-76/H-04601 to 04604.
In welded structures operating in the
aqueous media containing, e.g., alkaline solutions, soon after the installation
has been introduced to use, the cracks of orientation transverse to the weld
appear and are penetrating to the parent metal. It has been observed that
corrosion of this type may take place only under the combined effect of
mechanical stresses and chemical reaction of the hydroxide solution. Therefore,
to arrest the development of corrosion, the welded joint should be
stress-released by annealing at a temperature of 650oC, or possibly
by subjecting it to the thermal and mechanical treatment. The water preheaters
in modern power boilers and pipe systems through which the aggressive media are
flowing are composed of the pipe sections joined together by welding. Often in
these joints some perforations appear due to the selective corrosion which
usually occurs near the weld end.
To avoid corrosion of this type, it
is recommended to make the girth weld with several runs instead of one single
run, laying the runs in such a way as to make the beginning of the new run and
the end of the previous one shift in respect of each other.
The more numeous are the runs, the
thinner is the electrode, and the lower is the welding current, the more
reliable is the joint. The welded joints in pipes expected to operate under the
conditions of pressure should be, in principle, subjected to heat treatment.
For the construction of some pipelines,
specially those handling potable water and water used by food industry,
chromium steels of the 0H13, 0H13J, 0H17 and 0H17T grades are commonly used
nowadays, though the austenitic 0H18N9 and 1H18N9 grades are also observed to
gain popularity. These steels are usually welded with electrodes giving the
weld metal of an austenitic structure in grades E 13 B 22, E 17 B 22, E 19 9 R 22 or E 19 9 B 22 (acc. to EN 1600).
In some environments, the welded joints of these steels are susceptible to
intercrystalline and stress corrosion.
If the welded joints in the above
mentioned steels are expected to offer some resistance to the intercrystalline
corrosion, they should be subjected to heat treatment which consists in
annealing at a temperature of 800oC for 2 hours (when the weld has
been made with austenitic electrodes), or 4 hours (when a ferritic filler has
been used), followed by slow cooling together with furnace to a temperature of
600oC and rapid cooling in the air, next. The necessity to use so
complex heat treatment seriously limits the range of application of these
steels in construction of chemical apparatus, specially because of the grain
growth, and hence higher brittleness of the material.
The risk of occurrence of the intercrystalline corrosion in a welded
joint depends on the carbon content in welded steel and in metal deposited from
the electrode used for welding; it also depends on the heat volume supplied in
a time unit. To produce welded joints resistant to the intercrystalline
corrosion it is recommended to use the steel of low carbon content or steel
stabilised with titanium. If the joint should be deposited with several runs,
the layer of the weld contacting directly the aggressive medium should be made
with an electrode of low carbon content (0,02...0,03%C), or it should be
stabilised with, e.g., niobium.
The knife-line corrosion attack is
of an intercrystalline nature and it occurs specially in welded joints made
from the chromium-nickel-molybdenum steels. Corrosion of this type occurs
mainly at the welded joint-parent metal interface, and it may destroy in a
relatively short time the whole joint. As preventive means it is recommended to
use electrodes of low carbon content, possibly electrodes that will give the
weld metal of a ferritic-austenitic type, or to apply a stabilising heat
treatment..
The
welded joints made in chromium-nickel and chromium-nickel-molybdenum steels
with the commonly used electrodes are less resistant to stress corrosion than
the parent metal. In some cases, the resistance of the welded joint to the
stress corrosion is only 10% of the parent metal resistance.
PAD WELDING
Pad welding is the process of application of metallic coatings where a
characteristic feature is remelting of
the substrate material on which
the padding weld has been deposited.
When for pad welding the material of
properties different than the properties of parent metal is used, there are
some side effects resulting from mixing of the base (pad welded) metal with the
fused padding metal. The degree to which these two materials get mixed together
is the greater, the greater is the depth of penetration of the padding weld
into the base metal.
The content of carbon in the individual layers of a padding weld depends
on the content of carbon in parent metal and in the filler, and also on the
penetration depth. Therefore, to obtain the highest possible corrosion
resistance, both parent metal and filler metal should have the lowest possible
content of carbon with the highest possible content of elements, like chromium
and nickel.
Among the numerous methods available, the smallest penetration depth
into the pad welded metal gives the process of pad welding with shielded arc and
strip electrodes, the highest – the pad welding in protective gas atmosphere
with consumable electrodes, and with shielded arc and one wire electrode.
Pad welding with strip electrodes is
widely used by industry in the manufacture of
heavy-wall tanks (e.g. for the nuclear power industry), which should
have a very high corrosion resistance in their inside part.
METHODS TO TEST THE CORROSION
The practical aim of corrosion testing
is to determine the life of metals or alloys during performance under given
conditions. The results of these tests should give an answer to the question in
what way the metals or alloys will behave on performance.
At present, the following tests are
conducted:
1.
Laboratory tests under the conditions
created artificially by means of various devices.
2.
Field tests under actual operating
conditions.
3.
Laboratory tests carried out on real
facilities and under the real operating conditions.
Numerous methods of corrosion
measurement are available, but they all can be divided in qualitative and quantitative methods.
QUALITATIVE METHODS consist in
observations of the object exposed to corrosion and are divided into:
a)
direct (visual observations with
unarmed eye)
b)
macroscopic (observations under
magnifying glass)
c)
microscopic (observations under
microscope)
d) evaluation of changes in mechanical properties
All these methods are characterised by
great simplicity. The last method is very easy and handy in determination of
the internal (hidden) corrosion.
QUANTITATIVE METHODS
These methods consist in the determination of:
a)
the time when on a given surface the first corrosion spots appear and the
corrosion spots count in a time unit;
b)
the change in specimen thickness and depth of pits, or loss of weight;
c)
increase of weight when the corrosion products are insoluble and stick to the
specimen. If this is the case, the corrosion
is expressed as an increment in
weight in a time unit, e.g. g/m2·year.
d)
mechanical properties during the tensile test (Rm and A), or
technological properties during the bending test (the number of flexions). In
this case the corrosion is expressed as a percent drop in the value of the
tested property during one year.
Besides the tests mentioned above,
there are also numerous other, often very complicated, tests, like the
determination of the volume of the soluble corrosion products in a solution, of
the volume of evolved hydrogen or absorbed oxygen, changes in electric
resistance, changes in the thermal effect, changes in the ability to reflect
light, etc.
OWN INVESTIGATIONS
TESTING
OF THE PADDING WELDS CORROSION RESISTANCE
Testing of corrosion resistance was carried out on the steel specimens
with and (for the sake of comparison) without padding welds.
The aim of the investigations was to evaluate the behaviour of pad
welded elements (the pad welded specimens) under the performance conditions. To
achieve this goal, simulation tests were carried out under the laboratory
conditions.
Two methods of the evaluation were
applied:
1)
visual – the evaluation of changes in the appearance of the specimen surface,
2)
evaluation of changes in the specimen weight and dimensions.
Evaluating the surface condition, it is necessary to evaluate the type
and colour of the corrosion products, the severity of corrosive attack, and the
symptoms of corrosion. The qualitative evaluation should indicate the type of
corrosion, its character and if there are any local damages caused by the
corrosion or not.
TEST MateriaL FOR INVESTIGATION OF THE CORROSION RESISTANCE OF PADDING
WELDS
Corrosive environment:
water; aqueous solutions of H2SO4,
HNO3, HCL,
NaCl,
simulating the saturation of water from the abyssal or geothermal springs with
the above mentioned compounds.
Basing on the reference literature and
the results of own investigations it has been assumed that the 5% concentration
of corrosive media is corresponding with great probability to the real
conditions of operation of a steel (metal) structure, e.g. of a pipeline for
handling of the abyssal and geothermal waters, taking into account the factors,
like water temperature, flow rate, gas saturation ratio, and erosive wear
during an uninterrupted operation for 25 years.
Table 1. Chemical composition of steel S235JR
(St3S);
in accordance with a
standard EN 10025: 1993
C
|
Mn
|
Si
|
P |
S |
Cr |
Ni |
|
0÷0,22 |
0÷1,1 |
0,1÷0,35 |
0÷0,05 |
0÷0,05 |
0÷0,3 |
0÷0,3 |
Table 2. Chemical composition of weld metal [%]
|
Designation |
Type |
C |
Si |
Mn |
Cr |
Ni |
Mo |
|
1 |
E19 9 B 22 |
0,07 |
0,3 |
1,2 |
19,5 |
9,0 |
|
|
2 |
OK16.31 |
0,03 |
0,6 |
1,1 |
19,0 |
12,0 |
2,5 |
|
3 |
OK 61.30 |
0,03 |
0,8 |
0,6 |
19,0 |
10,0 |
0,75 |
|
4 |
OK 12.51 |
0,1 |
0,9 |
1,5 |
|
|
|
|
0 |
No weld |
||||||
Table 3. Corroding medium.
|
Designation |
5% of water solution |
|
S |
H2SO4 |
|
N |
HNO3 |
|
H |
HCl |
|
C |
NaCl |
TEST METHODS
Tests and investigations were carried
out on the specimens of S235RJ steel. On some of the specimens, the padding
welds were deposited with wire electrodes and with coated electrodes, following
guidelines adopted in the research program. The padding welds were deposited
semi-automatically under gas shielding and manually with coated electrodes (MAG
proccess).
PARAMETERS
OF THE PAD WELDING PROCESS
Pad welding under gas shielding:
Arc
voltage - U1=18,3 [V],
Welding current - I1=128 –
132 [A]
Wire
feed rate - ve=3,45 [m/min], Protective gases - CO2, Argon
Manual pad welding with coated
electrodes:
Electrode
diameter - 4,0 [mm], Welding current: 114
[A]
PROGRAM OF RESEARCH
On S235JR steel plates, altogether 16
padding welds were deposited – four with each type of the electrode and wire,
and then the pad welded specimens and the four specimens without padding welds
were subjected to the attack of aqueous solutions of H2SO4,
HNO3, HCL and NaCl.
Table 3. Project of tests in accordance with the table 2 - 3
|
Weld metal |
Corroding medium |
|||
|
H2SO4 |
HNO3 |
HCL |
NaCl |
|
|
E 19 9 B 22 |
1S |
1N |
1H |
1C |
|
OK. 16.31 |
2S |
2N |
2H |
2C |
|
OK 61.30 |
3S |
3N |
3H |
3C |
|
OK 12.51 |
4S |
4N |
4H |
4C |
|
No weld |
5S |
5N |
5H |
5C |
(Detali of the diagram above)
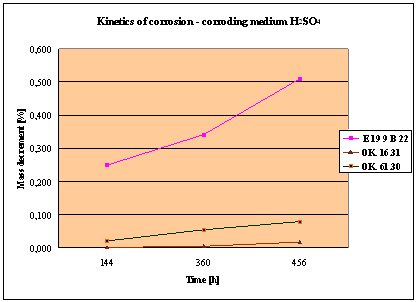
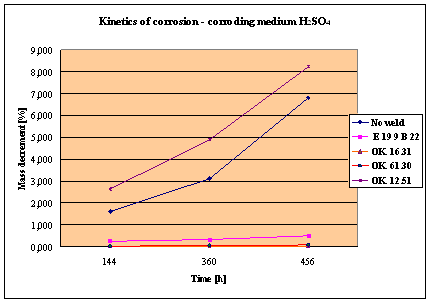
Fig. 1. Mass decrement of surfaced plate specimen; water solution H2SO4
(Detali of the diagram above)
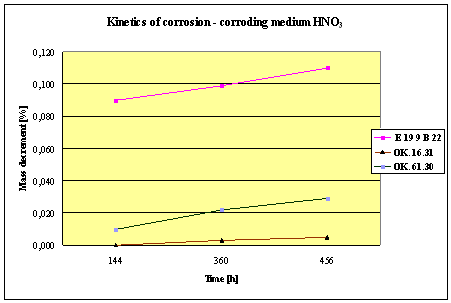
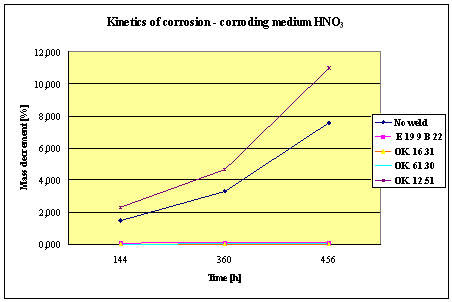
Fig.2. Mass decrement of surfaced plate specimen; water solution HNO3
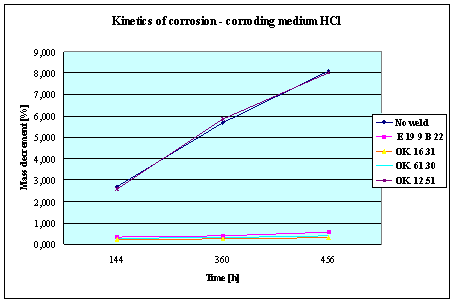
(Detali of the diagram above)
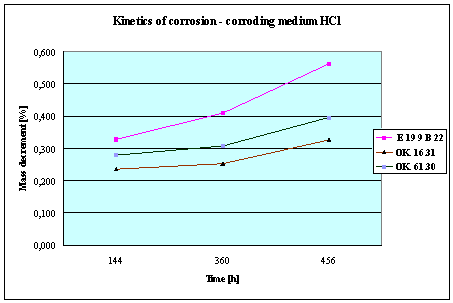
Fig.3. Mass decrement of surfaced plate specimen;
water solution HCl
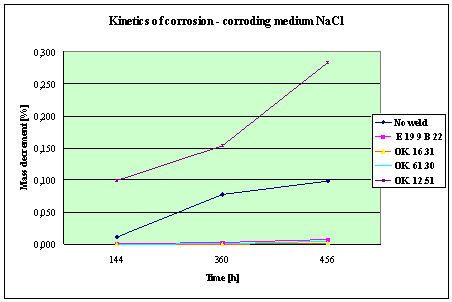
(Detali of the diagram above)
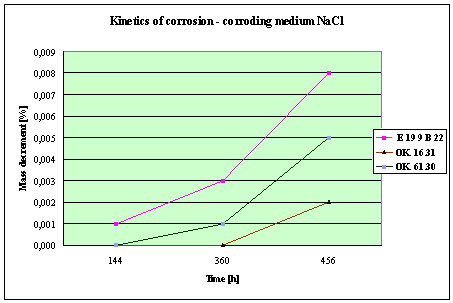
Fig. 4. Mass decrement of surfaced plate specimen;
water solution NaCl
THE RESULTS
AND CONCLUSIONS
1.
The corrosion resistance of the steel
and padding welds depends on the type of the
corrosive medium (the contamined water).
2. The
pad welding with wire electrodes, which give the weld metal of the same
properties as the common steel, makes no sense. As results from the plotted
graphs, the corrosion resistance of such padding welds is lower than that of
the steel itself.
3. The
highest corrosion resistance had the specimens with padding welds deposited with
an Autrod OK. 16.31 wire electrode containing 2,5 % Mo. The results almost
identical were obtained when electrodes giving the weld metal of much lower Mo
content were used, which is quite important from the economic point of view.
4. Slightly
inferior results were obtained in the case of padding welds deposited with E 19
9 B 22 (ES 18-8 B) electrodes. The drop of corrosion resistance is due to the
effects that accompany the reactions taking place between the components
of the electrode coating (specially if
they are of a basic reaction) and parent metal. An important factor improving
this resistance is without any doubt the exact removal of slag originating from
the melted electrode coating and periodical (according to the technical
possibilities) cleaning of the padding welds or welded joints.
5. The
greater losses – independent of the type of the padding weld – occurred when
water was acidified with sulphuric acid and with hydrochloric acid, which is
probably due to depolarisation and its type related to either oxygen or
hydrogen.
6. It
seems advisable to use the technology of pad welding in structures and
facilities that should offer high corrosion resistance, specially as regards
current repairs and emergency repairs (the latter ones being of major
importance), instead of replacing the whole sub-assemblies. Pad welding is
fully justified from the point of view of economy. It provides, moreover, some
opportunities to reduce the volume of the accumulating waste parts.