K.Bekmyrza, M.K.Myrzakhmet, M.Nikl*, V.Jery*, K.S.Baktybekov
L.N.Gumilyov Eurasian National university, Astana,
*Institute of Physics of Academy
of Sciences of the Czech Republic, Prague
ABOUT LUMINESCENT PROPERTIES OF
MONOCRYSTALS OF THE DIFFICULT SULPHATES ACTIVATED BY THALLIUM
Introduction
Crystals
of sulphate of ammonium and the mixed crystals of sulphate of potassium and
lithium are interesting with some
phases caused by various behavior of cations and annions at various
temperatures.
Ammonium
sulphate at 223 K to turns from the high-temperature paraelectric form I in
ferroelectric phase II [1]. Both forms are ortorombical, group forms I Ðïàò, and forms II — Ðïà21 is spatial. Under a condition for trimetric
elementary cells b > à > ñ with, the axis c
with in a phase II is a direction spontaneous polarization. The phase I
possesses three mirror planes àb, bc and àñ and the inversion center, and the phase II doesn't
have center of inversion and a reflection plane àb.
In both
forms, paraelectric and ferroelectric,
for ammonium ions in a lattice there is environment of two types. Types of ions
corresponding to them are designated as NH4 (I) and NH4
(II [1]. Ion NH4 (I) has five sulphates-ions in the nearest
environment, and ion NH4 (II) — six (in both phases). Ions NH4
(I) are less mobile, than ions of the second type. With increasing
concentrations of potassium ions, the transition temperature decreases and activation energy of the ions NH4 (I) decreases. Moreover, last type of ions is mainly replaced, and in
enough enriched ions potassium the mixed crystals there are only ions NH4
(II). From measurements of time of a relaxation on a phase I of salt (NH4)2S04
follows that above 300 K ammonium ions
begin to diffuse in a lattice with energy of activation 75 kj/mol [2].
Transition
II→I partially smooth as thermal capacity measurements have shown. It is
accompanied by the gradual reduction of volume reaching as a result
approximately 1,5%, thus compression is concentrated only along an axis and
[3]. However, though transition is also stretched on an interval approximately
in 50 K, it comes to the end nevertheless isothermally. Thus, in the Curie
point sharp change of spontaneous
polarization [3] and factors of an elastic pliability [4] is observed. Nevertheless, there is no a sharp change in
frequencies of librational (torsional)
oscillations, that has been established by long-wavelength infrared spectra [5], infrared
spectra and Raman scattering
[6], nor in the
effective cross section of neutron
scattering.
For
ammonium sulphates, and also the mixed sulphates of potassium and the lithium
activated by thallium, remain unsolved
questions:
•
distinction of optical properties of ions Tl+ for two various
positions of potassium with which they replace,
•
optical properties of pair centers Tl+2 in these
crystals.
Experiment
Crystals
of sulphate of ammonium and the mixed sulphates of lithium and potassium pure
and in the presence of a thallium impurity are grown up from a water solution
by a method of slow evaporation at room temperature at the Eurasian national
university of L.N.Gumilyov. Measurements of optical properties of these
crystals are carried by the equipment of department of optical materials of
Institute of Physics of Academy of Sciences of the Czech republic in Prague.
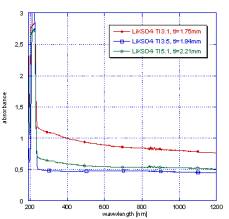
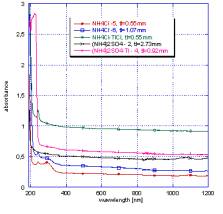
Absorption
of crystals was measured at room temperature by means of installation Shimadzu
3101PC, a luminescence in a wide range of temperatures from temperature of
liquid nitrogen to 500°Ñ – with help
spectrofluorimeter HJY 5000M.
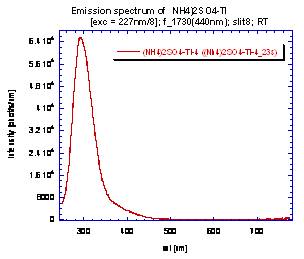
In fig.
1 - 2 are shown the comparison with standard scintillator BGO spectra X-ray all
measured by us it is exemplary. The samples activated by thallium always show
more intensive strip of radiation with various intensity (depending on the form
of the sample, concentration of thallium, quality of a material).