The Kazakh national university after al-Farabi, Almaty, Kazakhstan
diastereoselective
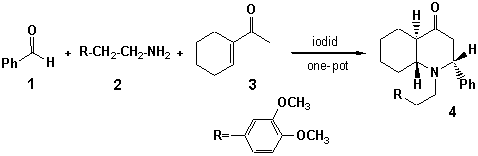
synthesis of 1-[2-(3΄, 4΄- dimethoxy-phenyl)ethyl]-2-phenyldecahydroquinoline-4-ONE
Last years, the chemistry of
decahydroquinoline alkaloids has received a new impulse in the development. The
literary data on biological activity of decahydroquinoline alkaloids, and also
the experience stored by this time on their synthesis opens prospects for
creation of new more effective medical preparations products with directional
effect and agrochemicals.
decahydroquinoline backbone is a structural
basis of many natural alkaloids (lepadines, pumiliotoxins at al.) in possession
of different biological activity. Introduction in to decahydroquinoline
skeleton the pharmacogenetic fragments often meeting in structure of natural
alkaloids and working out of the directed synthesis of new highly effective
biologically active compounds is an actual problem of modern organic and
bioorganic chemistry.
Such approach used at
preparation of 1-[2-(3΄, 4΄- dimethoxyphenyl)
ethyl]-2-phenyldecahydroquinoline-4-one (4).
At a choice of amine a natural alkaloid, close by structure, – messine - 2 (3 ', 4 ', 5
'-trimethoxyphenyl) ethylamine has been accepted as a basis. The combination in
one molecule of decahydroquinoline skeleton and dimethoxyphenylethyl group in the synthesised compounds do them
close structural analogues of such natural alkaloids and spasmolytic
aslobinaline, salsolidine, papaverine, no-spa, etc. [1-8]. Earlier the
synthesis of this decahydroquinolone (4) is carried out by heterocyclization of
styryl-1-cycklohexenylketon with homoveratrylamine (2) in absolute ethanol. As
a result of reaction a mix of a trans-
and cis-aminoketones is produced,
yield 67 %, with considerable prevalence of a trans-isomer in which the phenyl group at С2 is focused
equatorially. The content of a cis-isomer
about 2 % [9,10].
For the purpose of yield
increase it is carried out one-stage diastereoselective synthesis of this
aminoketone (4) by stirring of equimolar mix of benzaldehyde,
homoveratrylamine and acetylhexene at room temperature in presence of trace of
iodine. As a result the trans-isomer
of 1-[2-(3΄, 4΄- dimethoxyphenyl)
ethyl]-2-phenyldecahydroquinoline-4-one (4)
with yield 91 % is obtained.
The structure of aminoketone 4 is determined with the help of IR- ,
a nuclear magnetic resonance (NMR) 1Н spectroscopy.
In
IR-spectra of stereoisomer 4, there are intensive absorption bands in the
region of 1700-1715 sm-1, characteristic to valence fluctuations of
carbonyl group.
In NMR 1Н spectra of stereoisomer 4 it
is found out a weak field signal in area d 3,86 ppm, (except for signals
of protons of aromatic rings), which is caused by proton Н2 and observed in
the form of a doublet (2J 12,5 Hz) and doublets (2J 2,4
Hz). Vicinal constants testify to an equatorial arrangement of phenyl at С2. Though signals of
angular protons also get to resonance area methylene protons of substitute at
nitrogen, the signal structure at С9 however is distinctly shown, on which it is possible
to judge a way of a joint of cycles. This signal represents a triplet (2J
10,0 Hz), doublets (2J 3,2 Hz) that is characteristic for a trans-joint of cycles.
Table 1. NMR 1Н spectra of decahydroquinolone-4
|
Index of
compound |
d, ppm in CDCl3 |
КССВ, J, Hz |
||||||
|
Н2 |
Н3а |
Н3е |
Н9 |
Н10 |
Н2Н3а |
Н2Н3а |
Н3аН3е |
|
|
4 |
3,86 |
2,74 |
2,45 |
2,51 |
2,32 |
12,5 |
2,4 |
13,8 |
On the basis of the above-mentioned data to aminoketone 4 the following configuration is attributed:
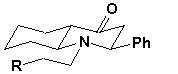
If heterocyclization of
styryl-1-cycklohexenylketon with homoveratrylamine, earlier carried out by us,
led to formation of a mix of trans-
and cis-aminoketones, with
considerable prevalence of trans-isomer,
one-stage three-component synthesis goes stereo-directly with formation only
one stereoisomer with a trans-joint
of cycles and equatorial orientation of phenyl group at С2.
references
1.
Robison M.M., Lamber B.F.,
Dorfman L., Pierson W.G. The stereochemistry and synthesis of the Lobinaline
ring systhem // J.Org.Chem.1966. V. 31. N 10. P. 3220-3223.
2. Dali J.W., Witkop B.,Tokuyama T., Nishikawa T., Karle
I.L.
/ Gefirotoxins, histrioniotoxins and pumiliotoxins from the neotropical frog
Dendrobates histrionicus // Helv. Chim. Acta. 1977.V.60. P.1128-1140.
3.
Dali J.W., Mc Neal E.A.,
Overman L.E., Ellison D.H. B. A new class of cardiotonic agents; structure agents;
structure - activity correlation for natural and syntetic analogues of the
aikaloid pumiliotoxin B // J.Med.Chem. 1985. V.28. № 4. P.
482-486.
4. M. Mena and J. Bonjoch // Model studies in the
lepadin series: syntesis of enantiopure decahydroquinolines by aminocyclization
of 2-(3-aminoalkyl)cyclohexsanones. Tetrahedron 2005. 61. 8264-8270.
5. C. Gravier-Pelletier, W. Maton, G. Berto and Y.Le
Merrer // Synthesis and glicozilas inhibitory activity of enantiopure
polyhydroxylated octahydroindoles fnd decahydroquinolines, analogs to
castanospermine. Tetrahedron 2003. 59. 8721-8230.
6. J.W. Daly, H.M. Carraffo, P. Jain, T.F. Spande, R.R.
Snelling, C. Jamillo, A.S. Pand // Arthropod-frog Connection: Decahydroquinoline
and Pyrrolizidine Alkaloids Common to Mycrosympatic Myrmisine Ants and
Dendrobatide Frogs. Journal of Chemical Ecology. 2000, 26:1, 73-85.
7. T.H. Jones, J.S.T. Gorman, R.R. Snelling, J.H.C.
Delabie, M.S. Blum, H.M. Garraffo, P. Jain, J.W. Daly, T.F. Spande // Further
Alkaloids Common to Ants and Frogs: Decahydroquinoline and Qyinolizidine.
Journal of Chemical Ecology. 1999, 25:5, 1179-1193.
8.
Машковский М.Д. Лекарственные средства. Ч. I. М.: Медицина, 1988.
С.624.
9.
Жилкибаев О.Т. Синтез и стереоизомерия 1-[2-(3¢,4¢-диметоксифенил)этил]-2-фенил- и 1-[2-(3¢,4¢-диметокси-фенил)этил]-2-(3¢,4¢-диметоксифенил)дека-гидрохинолонов-4// «Химия
природных и синтетических биологически активных веществ». Труды ИХН МОН
РК – Алматы, 2004., Т.76.– С. 142–148.
10. Kozlovski V.I., Vdovichenko V.P.,Chlopicki S.,Malchik
S.S.,Praliev K.D.,Zcilkibaev O.T. // Antiarrhytmic profile and endothelial
action of novel decahydroquinoline derivatives. Polish Journal of Pharmacology
Pol.J.Pharmacolol., 2004, 56, 767-774.