Dvorkin L. J., doctor of technical sciences, professor, Bezusyak
O.V., candidate of technical sciences, associate professor, Kovalyk I.V.,
post-graduate student (National University of Water Management and Nature Resources Use,
Rivne)
THE EFFECT OF FOAM MULTIPLICATION
FACTOR ON DENSITY AND STRENGTH OF FOAMGYPSUM
The conditions of foam obtaining and its main
properties have been examined. The relations for the determination of
foamgypsum quality indicators depending on the foam multiplicity have been
obtained.
Foamgypsum is referred to as an effective building
material. It has small density, low thermal conductivity and high fire
resistance. The foamgypsum properties depend on the quality of its
consitituents, namely, on bonding foams and additives.
During our research, the foamgypsum G- 5-B-II VS V.2.7
B-82-99, of the SPE Helios Production under the trademark “FEROZIT” have been
used. As the surfactant, the foam solution was used, based on high molecular
olefinsulfonat - Hostapur OSB produced by “EuroChem 1”.
The aim of the research was to establish the influence
of the multiplicity of foam on the density and the strength of foam-gypsum.
Foaming process due to the joint influence of
physical, chemical and technological factors is rather difficult.
Foam depressiveness is substantially influenced by
physical and chemical properties of the solution (surface tension, density,
concentration of surfactant), the construction of technological apparatus,
regimes of technological process forming the foam, as well as external factors
(temperature, pressure, humidity, availability of dust) [1].
An important property of foam is its multiplicity
(foaming possibility of the solution) - the amount of foam which is expressed
in volume of foam, produced from the constant volume of solution, subject to
certain conditions during certain period of time. The factors affecting the
multiplicity of foam are the following: surfactant molecular structure, the
concentration of surfactant, temperature, pH and the solution surface tension.
Foam has been obtained through dispargation that is
mixing of solution foams and air. In technological aspect dispargation has been
conducted using mixer at 3000 nozzle revolutions per minute of within 2
minutes. The plan of experiment and the foam quality parameters are listed in
Table. 1.
In the experiment, the solution of dry substance
surfactants of 25 ml volume has been used. Solution concentration changed from
0 to 0.42%, and density - from 1 to 1.00011, g/cm3.
After dispargation, foam volume has been determined
through experimental method and the volume of gas in it - as the difference
between foam volume and the volume of solution:
![]() . (1)
. (1)
Foam
multiplicity b characterizes the relative content of gas and liquid in the
dispersed system:
![]() ,
(2)
,
(2)
where ![]() - the
amount of foam, cm3;
- the
amount of foam, cm3; ![]() - the
solution of surfactant, ml;
- the
solution of surfactant, ml; ![]() - the
volume of gas (air) in the foam, cm3.
- the
volume of gas (air) in the foam, cm3.
Taking into account equation (1) and (2), the amount of gas is
determined by the equation:
![]() . (3)
. (3)
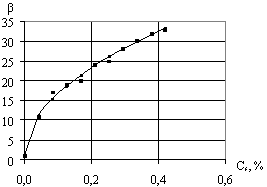
Fig.
1 shows experimental values
of foam multiplicity depending on concentration of surfactant solution. Basing on theoretical analysis of these
data, the equation has been suggested:
![]() , (4)
, (4)
where ![]() -
coefficient, which is determined experimentally depending on the properties of
surfactant solution;
-
coefficient, which is determined experimentally depending on the properties of
surfactant solution; ![]() -
surfactant dry matter concentration in the solution,%.
-
surfactant dry matter concentration in the solution,%.
Table 1
Experiment plan and foam quality characteristics
|
¹ |
Water weight, g |
Weight of SUF, g |
Volume of SUF solution ml |
Concentration of
SUF solution
% |
Density of SUF solution
g/cm3 |
Foam volume cm3 |
Gas volume
cm3 |
Foam
multip-licity b |
Foam density,
h/m3 |
|
1 |
25,000 |
0,000 |
25 |
0,00 |
1,00000 |
25 |
0 |
1 |
1,000 |
|
2 |
24,990 |
0,011 |
25 |
0,04 |
1,00001 |
281 |
256 |
11 |
0,085 |
|
3 |
24,979 |
0,021 |
25 |
0,08 |
1,00002 |
387 |
362 |
17 |
0,070 |
|
4 |
24,969 |
0,032 |
25 |
0,13 |
1,00003 |
468 |
443 |
19 |
0,051 |
|
5 |
24,958 |
0,042 |
25 |
0,17 |
1,00005 |
537 |
512 |
20 |
0,047 |
|
6 |
24,948 |
0,053 |
25 |
0,21 |
1,00006 |
597 |
572 |
24 |
0,044 |
|
7 |
24,937 |
0,063 |
25 |
0,25 |
1,00007 |
652 |
627 |
25 |
0,040 |
|
8 |
24,927 |
0,074 |
25 |
0,29 |
1,00008 |
702 |
677 |
28 |
0,035 |
|
9 |
24,916 |
0,084 |
25 |
0,34 |
1,00009 |
748 |
723 |
30 |
0,035 |
|
10 |
24,906 |
0,095 |
25 |
0,38 |
1,00010 |
792 |
767 |
32 |
0,031 |
|
11 |
24,895 |
0,105 |
25 |
0,42 |
1,00011 |
833 |
808 |
33 |
0,032 |
Foam density is defined as the ratio of foam mass to
its volume:
![]() , (6)
, (6)
where ![]() - weight
of solution and gas correspondingly, g;
- weight
of solution and gas correspondingly, g; ![]() -
density of gas (air),
-
density of gas (air), ![]() =
0.00129 g/cm3;
=
0.00129 g/cm3; ![]() -
solution density, g/cm3.
-
solution density, g/cm3.
The effect of foam multiplicity influence on its
density is shown in Fig. 2.
|
|
|
|
Fig.1. The dependence of foam
multiplicity on concentration of surfactant solution |
Fig.2. The influence of foam
multiplicity on its density |
During the formation of foamgypsum mass, it should be
noted that the multiplicity of foam should be viewed not in relation to dual
component systems (water + surfactants) but to multi-component systems (water +
surfactants + gypsum).
In the actual industrial process, different types of
additives (solidification retarders, superplasticizers, fiber, etc.) are being
added to these basic components.
In the experiment, the foamgypsum mass has been got
with the use of traditional technology [2].
Three series of experiments have
been conducted with W/G equal to 0.55, 0.58 and 0.61. The mass of gypsum binder
was constant. The volume of gas varied from 0 to 808 cm3. The
samples 10 × 10 × 10 cm have been formed of the foamgypsum mass.
After hardening and taking off the casing, the samples were subject to drying
to the constant mass at the temperature of 55 ° C. Then their weight and
strength have been determined.
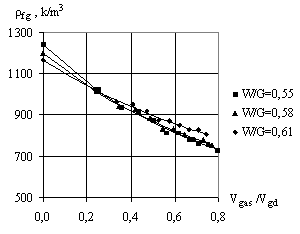
When the foamgypsum mass is being generated, its
volume depends on the volume of gypsum bonding, amount of water and gas. So, in
the study of gypsum density it is important to introduce the complex factor, which
takes into account the impact of each of these factors:
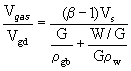 , (7)
, (7)
where ![]() - the volume of gypsum paste;
- the volume of gypsum paste; ![]() - the actual density of gypsum binder,
- the actual density of gypsum binder, ![]() = 2.65 g/cm3;
= 2.65 g/cm3; ![]() - density of water. The results of determination of foamgypsum
density
- density of water. The results of determination of foamgypsum
density ![]() depending on
depending on ![]() factor are presented in Fig. 4. To determine the average
foamgypsum density we have suggested the equation:
factor are presented in Fig. 4. To determine the average
foamgypsum density we have suggested the equation:
![]() ,
(8)
,
(8)
where ![]() - the
true density of gypsum,
- the
true density of gypsum, ![]() = 2.30
g/cm3;
= 2.30
g/cm3; ![]() - the
experiment coefficient, which takes into account the degree of gas reduction as
a result of pressure of the mass of gypsum paste on it. If W/G = 0.55 -
- the
experiment coefficient, which takes into account the degree of gas reduction as
a result of pressure of the mass of gypsum paste on it. If W/G = 0.55 - ![]() = 0875,
if W/G = 0.58 -
= 0875,
if W/G = 0.58 - ![]() = 0762,
if W/G = 0.61 -
= 0762,
if W/G = 0.61 - ![]() = 0597.
= 0597.
This equation adequately describes the experimental
points in the research. When ![]() <0.3, as you see from Fig. 3, the water-gypsum ratio has a significant influence on the density of
foamgypsum.
<0.3, as you see from Fig. 3, the water-gypsum ratio has a significant influence on the density of
foamgypsum.
That is, with increasing of W/G the foamgypsum porosity
increases and its density decreases. When ![]() > 0.3, the volume of gas makes a significant impact on the foam
gypsum density. But with the increase of W/G gypsum paste mass increases, which
leads to the reduction in gas volume and increasing the foam gypsum density.
> 0.3, the volume of gas makes a significant impact on the foam
gypsum density. But with the increase of W/G gypsum paste mass increases, which
leads to the reduction in gas volume and increasing the foam gypsum density.
The foamgypsum strength depends only on its density.
Therefore, it is advisable to introduce factor ![]() , that is, ratio of average foam gypsum density
to the true gypsum density,
, that is, ratio of average foam gypsum density
to the true gypsum density, ![]() = 2.3
g/cm3. Fig. 4 shows the value of
foamgypsum strength according to the authors and H. Bruncker data, depending on the
= 2.3
g/cm3. Fig. 4 shows the value of
foamgypsum strength according to the authors and H. Bruncker data, depending on the
![]() ratio.
ratio.
The processing of experimental data has allowed to
obtain an equation for predicting the foamgypsum strength according to the ![]() factor:
factor:
![]() , (9)
, (9)
where ![]() - the
coefficient that corresponds to the strength of gypsum if the pores are
suggested. The equation (9) adequately describes the authors’ data when
- the
coefficient that corresponds to the strength of gypsum if the pores are
suggested. The equation (9) adequately describes the authors’ data when ![]() = 10.5
MPa and Bruncker’s data when
= 10.5
MPa and Bruncker’s data when ![]() = 6.4
MPa
= 6.4
MPa
|
. |
|
Fig. 3. Dependence of
the average foamgypsum density on the ratio of plaster paste volume |
|
|
|
Fig.
4. Dependence of foamgypsum strength on the relation of foamgypsum density to the
true density of gypsum: 1
– authors’ data, 2 - H. Bruncker’s data [3]. |
Thus, on the basis of
experimental studies, the equations for predicting the strength of foam and the
strength of foam-gypsum have been obtained. They can be used in the design of
foam-gypsum composition.
Literature:
1. Òèõîìèðîâ Â.Ê. Ïåíû. Òåîðèÿ
è ïðàêòèêà èõ ïîëó÷åíèÿ è ðàçðóøåíèÿ 2-å èçä., ïåðåðàá. – Ì.: Õèìèÿ, 1983. –
264 ñ.
2. Ðóæèíñêèé Ñ. È äð. Âñå î ïåíîáåòîíå. – 2-å èçä.,
óëó÷øåííîå è äîïîëí. – Ñïá, ÎÎÎ «Ñòðîé Áåòîí», 2006, 630 ñ.:èë.
3. Ãèïñ: Èçãîòîâëåíèå è ïðèìåíåíèå ãèïñîâûõ
ñòðîèòåëüíûõ ìàòåðèàëîâ.: Ïåð. ñ íåì./Õ. Áðþíêåð, Å. Äåéëåð, Ã. Ôèò÷ è äð.; Ïîä
ðåä. Â. Á. Ðàòèíîâà. – Ì.: Ñòðîéèçäàò, 1981. – 223ñ.