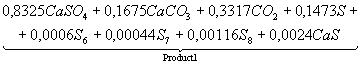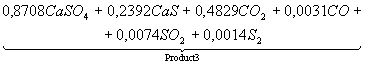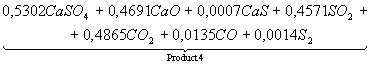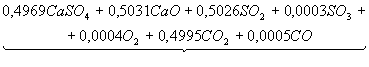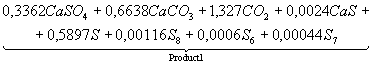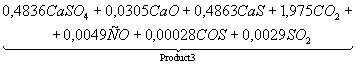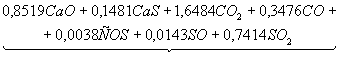Yerubay A.A., Bishimbayev V.K., Shevko
V.M.
Ph.D
student, Dr of Technical Sciences.
M.Auezov South-Kazakhstan
State University
Shymkent, Kazakhstan
Thermodynamic
modeling of interaction of calcium sulfate with carbon oxide (II)
For processing large-tonnage
production wastes of extraction phosphoric acid that is phosphogypsum some
variants of producing phospholime from it have been offered.[1-3] It is shown
by semi-industrial tests, that the degree of phosphogypsum decomposition in
rotating tube furnaces and fluidized-bed furnaces comprises 94- 97%. Formed by
this phospholime contains 58-76% of ÑàÎ. Process of
phospholime production from phosphogypsum now is not yet fully perfect, as the
content of CaS and CaSO4 in the product comprises accordingly 0,6-1,4
and 0,8-4,9%.
The task of the present work
was to determine optimum parameters of CaSO4 decomposition in
presence of CO as applied to agglomeration processing of phosphogypsum,
providing incomplete oxidation of natural coal carbon.
Research was carried out by
the method of thermodynamic modeling and a method of mathematical planning with
further optimization of technological parameters on the computer. For
thermodynamic modeling the program complex “Astra” developed in N.E. Bauman Moscow
High Technical School was used. Fundamental principle of a maximum entropy is
put in the basis of algorithm.[4,5] This method gives a unique opportunity of
the generalized description of any high-temperature state with the help of
thermodynamics fundamental laws, irrespective of conditions and ways of
achieving equilibrium. The database of the program complex Astra contains
information on thermodynamic properties of 5547 individual substances
systematized in Institute of high temperatures of the Russian Academy of
Sciences and the USA National bureau of standards.[6,7] The information
received by means of the program complex “Astra” allows to determine the
equilibrium degree of initial system elements transition into products of
reaction, and also composition of gas and condensed phases. Influence of
temperature (Ò from 500 to 1800Ê)
was investigated and distinct from [8,9] influence of a number of moles in
system CaSO4 – nCO (n from 0,2 up to 2,5) on the equilibrium
degree of Ñà distribution, including its
distribution in ÑàÎ was studied.
The carried out research has
shown, that the interaction happens with participation of 22 substances: CaSO4,
CaCO3, CaO, CaS, CO, CO2, COS, CS2, S, S2,
S3, S4, S5, S6, S7, S8,
SO2, SO3, SO, S2O, O2, O in system
CaSO4 – nCO depending on
temperature and number of CO moles. In figure 1 the information about influence
of temperature and number of CO moles on the degree of (α) calcium
distribution is given from which it follows that the basic calcium containing
compounds in the system are CaSO4, CaCO3, CaS and CaO.
From (Figure 1) it follows that in examined systems irrespective of CO moles number
in a temperature interval 500 – 600K
CaCO3 is being formed. The degree of formation (for example at Ò = 500Ê) CaCO3 grows with increase
of CO moles number in the following sequence:
|
n |
0,2 |
0,45 |
1,0 |
1,55 |
1,8 |
2,5 |
3,0 |
|
αCaCO3,
% |
6,62 |
14,88 |
33,30 |
51,43 |
58,44 |
89,97 |
98,82 |
Thus dependence ![]() looks like:
looks like:
![]() (1)
(1)
for which the criterion of suitability of approach R2 (factor of determination) comprises 0,9997.
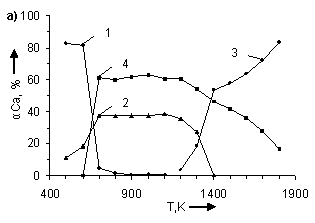
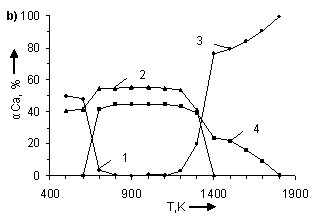
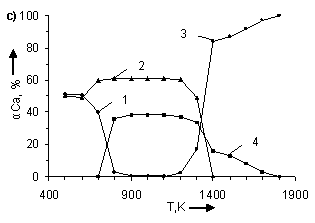

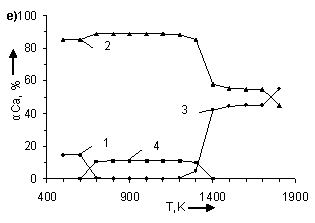
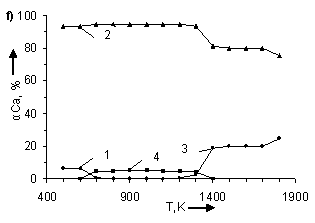
Figure. 1. Influence of
temperature and CO moles number (n)
in system CaSO4 – nCO on
the degree of calcium distribution (α) at pressure 0,1MPa. Where: 1 – CaCO3;
2 – CaSO4; 3 – CaO; 4 – CaS. In figures a), b), c), d), e), f), g) CO moles number (n) varies as
follows: à) – n=2,5; b) – n=1,8; c) –
n=1,55; d) – n=1,0; e) – n=0,45; f) – n=0,2
CaS starts to be formed in
systems at Ò ≥ 600K. Moreover, with
the increase of CO moles number the degree of CaS formation and temperature
area of its existence grow. So αmaxCaS
grows as follows:
|
n |
0,2 |
0,45 |
1,0 |
1,55 |
1,8 |
2,5 |
3,0 |
|
αmaxCaS, % |
4,99 |
11,18 |
24,80 |
38,56 |
44,7 |
63,19 |
73,6 |
Dependence ![]() looks like:
looks like:
![]()
![]() . (2)
. (2)
ÑàÎ is formed in system at T > 1100K. The temperature of CaO formation start (ÒstartÑàÎ) depends on number
of CO moles in the system, changing (decreasing) as follows:
|
n |
0,2 |
0,45 |
1,0 |
1,55 |
1,8 |
2,5 |
3,0 |
|
ÒstartÑàÎ, K |
1237,3 |
1207,5 |
1166,2 |
1142,6 |
1136,5 |
1126,1 |
1116,3 |
Thus dependence ![]() looks like:
looks like:
![]()
![]() . (3)
. (3)

Figure. 2. Influence of
temperature and number of carbon oxide moles in the system CaSO4 – nCO on the degree of ÑàÎ formation (α)
at Ð=0,1ÌPa,where CO moles number (n) are specified at modeling
as follows 1-n=0,2; 2-n=0,45;
3-n=1,0; 4-n=1,55; 5-n=1,8; 6-n=2,5.
Appreciable formation of ÑàÎ in systems is being observed at T > 1300K. Moreover,
with the deviation of CO moles number from 1 it results in reduction of the
degree of ÑàÎ formation from CaSO4.
At n = 0,2 ÷ 0,45 the process is
being blocked and it does not get any development in a temperature interval
1400 – 1700K (Figure 2). At constant
temperature (for example 1600Ê),
close to technological temperature of agglomeration the influence of CO moles
number on αÑàÎ has an extreme
character:
|
n |
0,2 |
0,45 |
1,0 |
1,55 |
1,8 |
2,5 |
3,0 |
|
αCaO, % |
19,94 |
44,85 |
96,54 |
91,65 |
83,69 |
63,50 |
32,84 |
Influence CO on ÑàÎ is described by
the equation:
![]()
![]() . (4)
. (4)
The research was continued
employing rotortable plans of the second order to search optimum parameters of ÑàÎ production from CaSO4 in presence of CO.[10]
Temperature (Ò,Ê) and
number of CO moles (n) as applied to
reaction CaSO4+CO=CaO+CO2+SO2 were used as
independent factors. The degree of ÑàÎ (αÑàÎ, %) formation was the parameter of optimization. The
matrix of conducting the research and results of ÑàÎ formation, received by means of program complex Astra, are given in (Table
1) Using the program developed in SKSU (South-Kazakhstan State University) it
was defined, that the equations of regression αÑàÎ=f (T, nÑÎ) in the coded and natural scale have
accordingly the following types:
![]() (5)
(5)
![]()
![]() (6)
(6)
The received equation is
adequate, because the tabular value of Fisher’s criterion (6,59) is bigger than
the real value of this criterion (5,59).[11] On the basis of the received
equation of regression using the program complex MathCAD – 14 the volumetric picture
of the change of response surface (Figure 3) and its horizontal sections (Figure
4) was constructed.[11] As follows from (Figure 3), the surface of
the response has an extreme character. Moreover, the maximum of ÑàÎ formation should be expected in the technological
area limited by figure ABC (Figure 4), that is, at Ò from 1490 up to 1690K and n from 0,1 up to 1,49. The composition
of gas phase of the system in this case (at n = 1,1) depends on temperature (Table 2).
In technological area (1500 – 1700Ê)
the gas phase contains 49,79 – 49,84% ÑÎ2 and 49,87 – 49,88% SO2.
Table 1. Matrix of planning
research and results of the research of ÑàÎ production from
CaSO4 – nCO system at Ð = 0,1MPa
|
¹ No. |
Independent variables |
αCaO, % (researched). |
αCaO, % (calculated). |
|||
|
Coded type |
Natural type |
|||||
|
Õ1 |
Õ2 |
n |
Ò, K |
|||
|
1. |
-1 |
-1 |
0,45 |
1446 |
46,8 |
48,08 |
|
2. |
+1 |
-1 |
1,55 |
1446 |
84,6 |
85,95 |
|
3. |
-1 |
+1 |
0,45 |
1654 |
47,8 |
49,09 |
|
4. |
+1 |
+1 |
1,55 |
1654 |
96,0 |
97,36 |
|
5. |
+1,414 |
0 |
1,8 |
1700 |
78,0 |
76,63 |
|
6. |
-1,414 |
0 |
0,2 |
1400 |
17,2 |
15,72 |
|
7. |
0 |
+1,414 |
1,0 |
1700 |
99,8 |
98,46 |
|
8. |
0 |
-1,414 |
1,0 |
1400 |
91,3 |
89,68 |
|
9. |
0 |
0 |
1,0 |
1550 |
99,4 |
98,54 |
|
10. |
0 |
0 |
1,0 |
1550 |
99,0 |
98,54 |
|
11. |
0 |
0 |
1,0 |
1550 |
98,6 |
98,54 |
|
12. |
0 |
0 |
1,0 |
1550 |
98,7 |
98,54 |
|
13. |
0 |
0 |
1,0 |
1550 |
97,0 |
98,54 |
To define the reasons of the
low degree of ÑàÎ formation at small (0,5) and
high (2) CO moles number we also determined the distribution of S, O and C
except Ca. On the basis of this the equations of CaSO4 interaction
with various quantity of CO are given in (Tables 3 and 4).

Figure.
3. Influence of CO moles (n)
number and temperature (T) on the
form of the response surface - αÑàÎ, %
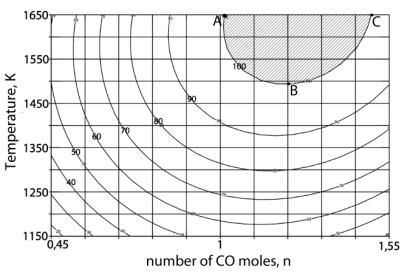
Figure. 4. Horizontal section
views of the response surface – αÑàÎ. Figures on lines –
αÑàÎ, %.
Table 2. Influence of
temperature on composition of the gas phase of CaSO4 – CO
system at Ð = 0,1MPa
|
Content of form. % |
Temperature, K |
||||||
|
1200 |
1300 |
1400 |
1500 |
1600 |
1700 |
1800 |
|
|
CO2 |
97,28 |
88,61 |
50,69 |
49,79 |
49,91 |
49,84 |
49,67 |
|
CO |
1,22 |
1,61 |
1,27 |
0,44 |
0,18 |
0,15 |
0,28 |
|
SO2 |
1,47 |
9,67 |
47,49 |
49,72 |
49,88 |
49,92 |
49,87 |
|
SO |
0,9*10-3 |
0,011 |
0,079 |
0,03 |
0,015 |
0,013 |
0,028 |
|
S2 |
0,008 |
0,069 |
0,42 |
0,8*10-3 |
- |
- |
- |
|
CaS |
0,012 |
0,021 |
0,022 |
0,2*10-3 |
- |
- |
- |
|
S |
- |
- |
0,8*10-4 |
0,2*10-4 |
- |
- |
- |
|
SO3 |
- |
- |
0,2*10-3 |
0,001 |
0,01 |
0,017 |
0,019 |
|
O2 |
- |
- |
- |
0,3*10-4 |
0,003 |
0,053 |
0,13 |
Table 3. The chemical
equations of interaction in system CaSO4 – 0,5CO
at Ð = 0,1MPa
|
Temperature, K |
Equations |
|
500 |
CaSO4+0,5CO
= |
|
700 |
Product
1 = |
|
1200 |
Product
2 = |
|
1400 |
Product
3 = |
|
1700 |
Product
4 = |
Table 4. The chemical
equations of interaction in system CaSO4 – 2CO
at Ð = 0,1MÐa
|
Temperature, Ê |
Equations |
|
500 |
CaSO4+2CO
= |
|
700 |
Product
1 = |
|
1200 |
Product
2 = |
|
1400 |
Product
3 = |
|
1700 |
Product
4 = |
It follows from comparison of
given (Tables 3 and 4), that not full CaO formation from system CaSO4
– nCO at surplus of CO is linked with
formation of irreducible CàS. When there is a lack of CO production CaO is
restrained by existence of CaSO4 in wide range.
If to lower pressure in the
zone of reaction it is possible to reduce ÒmaxÑàÎ in the system CaSO4 – CO. So, at Ð = 0,01ÌÐà ÒmaxÑàÎ = 1400K, at
Ð = 0,001MÐa –1300K. At presence of SiO2 in
phosphogypsum up to 17%, ÒmaxÑàÎ with formation of CaSiO3 at Ð = 0,001ÌÐa makes 1000K.
For phospholime this process is negative, as it reduces ÑàÎ activity. However for the subsequent production of
ferrumsilicocalcium from agglomerate, presence of SiO2 positively
influences CaSO4 decomposition.
On the basis of the carried
out research of interaction CaSO4 with CO it is possible to draw the
following conclusions:
- originally in system at Ò = 500K ÑàÎ is formed, and the
degree of its formation grows at increase of CO moles number;
- restoration of CaSO4
to ÑàÎ goes through a stage of CaS
formation;
- the degree of transition
CaSO4 to CaS grows at increase in CO system;
- ÒstartÑàÎ decreases from 1237,3K to 1116,3K at increasing the number of CO moles
from 0,2 to 3,0;
- ÒmaxÑàÎ (> 99,5%) at Ð = 0,1MÐa is being observed in a temperature interval
1500 - 1700K number of CO moles = 1,1
– 1,5;
- influence of temperature and
number of CO moles on αÑàÎ from CaSO4
has an extreme character;
- reduction of pressure from
0,1 to 0,001MÐa and input in
system SiO2 positively influence CaSO4 decomposition,
allow to reduce ÒmaxÑàÎ to 1000K.
References
1.
V.V. Ivanitskij, P.V. Klassen, A.L. Novikov Phosphogypsum and its use.
Moscow: Chemistry, 1990. 224 p.
2.
L.M. Razdorskih, S.V. Hrjashev, N.L. Solodjankina etc. Industrial tests
of process of thermochemical decomposition phosphogypsum on calcium oxide and
sulphurous gas in the furnace of a boiling layer. II Works of SRIFI (Scientific
institute of fertilizers and insect fungicides) «Use of phosphogypsum in a
national economy». Moscow 1983. Issue 243. p. 57-67.
3.
Technology of phosphoric and complex fertilizers / Under edition of S.D.
Evenchika and A.A. Brodskogo. Moscow: Chemistry 1987. 464 p.
4.
G.B. Sinjarev, N.A. Vatolin etc. Application of the COMPUTER for
thermodynamic calculations of metallurgical processes. Moscow: Nauka, 1982. 263
p.
5. B.G. Trusov, S.A.
Barak etc. the Automated system of the thermodynamic data and calculations of
equilibrium conditions // Mathematical methods of chemical thermodynamics.
Novosibirsk 1982. p. 213-219.
6.
JANAF Thermochemical tables, 2nd edition. NSROS-NBS US Gov
Printing Office, Washington, DC, 1971. 1141 p.
7.
Thermodynamic properties of individual substances. A reference book by
media in L.V. Gurvich, I.V. Vejtsa others in 4 volumes. Moscow: Nauka, 1982.
559 p.
8.
V.M. Shevko, E.YA. Kalashnikov, V.A. Êàpsaljamov
Opportunity reception ÑàÎ at interaction
ÑàSÎ4 with Í2, C, CO, ÑÍ4 // Works of the international
scientific – practical conference «Àuezov readings – 4»
and the third scientific conference of high schools of Southern region.
Shymkent 2004. p. 99-103.
9.
E.YA. Kalashnikov. Development of technology of reception calcium
carbide from phosphogypsum. A dissertation of
Candidate of technical sciences, Shymkent, 172 p. 2010.
10. S.A. Ahnazarov,
B.V. Kafarov Method of optimization of experiment with the chemical industry.
Moscow: the higher School, 1978. 319 p.
11. V.F.Ochkov. Mathcad
14 for students and engineers. St.-Petersburg.: BHV-Petersburg, 2007. 368 p.