Barbara Jachimko
University of Zielona Góra
Institute of Environmental Protection
Zielona Góra,
Poland
b.jachimko@iis.uz.zgora.pl
On the influence of meromixis on the chemical
composition of acid pit lake
Abstract: The aim of the research was to
evaluate the genesis of meromixis and the chemical composition development of
the biggest meromictic pit lake no 54 located within “antropogenic lake
district” in SW Poland. On the base of 27 years of monitoring, it was stated,
that the acidification of the lake has been still becoming larger, although the
pH value increased from 2.5 in the mixolimnion to above 4 in momimolimnion. The
two stages pattern of the chemical composition development was formulated: the
first period - intensive precipitation of iron salts, esp. K-jarosite in
miksolimnion (0-13 m depth) and dissolution plus ferrous reduction in
monimolimnion; the second period: due
to increase of pH value about 4,5 in monimolimnion, oversaturation this layer
due to alum salts, especially alunite. In the second period two buffering zones
are being developed: miksolimnion with iron salts precipitation, and
monimolimnion with the precipitation of aluminium salts.
Key words: acid pit lake,
meromixis, jarosite
1.
Introduction
Meromictic lakes –
that are lakes which do not mix completely each layer. This phenomena is not
common in natural lakes, but in mine pit lakes often happens. Three main
reasons of that are: steep slopes, relatively great depth and exposure to
highly mineralized inflows. There are the following mechanism of meromixis in
pit lakes development: a) formation of a less mineralized mixolimnion by inflow
of less mineralized water at the lake surface, b) enrichment of iron and sulfate due to the transport from
mixolimnion to monimolimnion of secondary minerals, c) influence of
sea water d) accumulation of substances in the monimolimnion due to biological
decay, e) evaporation f) influence of ground water of high TDS ((Boehrer,
Schultze 2000 and references there in). The deepest part of meromictic lake
water body – monimolimnion – has often very specific chemical composition: is
strong anoxia, rich of hydrogen sulfide and products of microbial decay.
Meromixis has a strong influence on the neutralization method of lake. It is
still open question how to recultivate the lakes in order not to destroy the meromixis, how it will be stable
after treatment and how the results are stable. The aim of the research was to
evaluate the genesis of meromixis and the influence on chemical composition of
the biggest meromictic pit lake no 54 within “antropogenic lake district” in SW
Poland.
2.
The area of study
“Antropogenic lake
district” is located along the polish-german border between
Trzebiel and Łęknica. It comprises about 100 reservoirs of different
size and chemical properties. The reservoirs were generated due to lignite
mining which started in the second half of the 19-th centaury and was lasting
till seventies of the 20-th centaury.
The research presented below, were focused on the biggest lake, numbered
54 (Jędrczak 1992) which comes from 1973 y (there are no exact data about
the rate and duration of filling up – fig. 1). The reservoir is surrounded by
strongly eroded heap of

![]()
Fig.
1 The area of study
excavation. The surrounding
area is covered by forest. Morphometry
of the reservoir is presented in Table 1. From the previous study it is known,
that the lake is extremely acid with high concentration of iron and sulphur.
The reservoir is classified to the meromictic type with relatively deep
monimolimnion (more than 10 m) (Solski, Jędrczak 1991a). The chemical
composition of its waters was presented earlier by (Solski, Jędrczak 1990;
Solski, Jędrczak, 1991b; Jędrczak, Jachimko, Najbar 1998).
Table 1 Morphometry of the reservoir no 54
|
Water level m above see level |
Surface area m2 |
Max length, m |
Max width, m |
extension coefficient |
Max depta, m |
Shore line lengh, m |
Shoreline development |
|
132,0 |
202.000,0 |
896 |
468 |
1,91 |
21,5 |
2625 |
1.65 |
The reservoir has no connections with surface waters. It results in the
fact, that the chemical composition of its water depends mainly on the
composition of ground water recharge and reactions within water body.
3. Methods
Water sampling
Water samples were taken in the deepest place of the lake from the
surface, 1-, 3-, 5-, 7-, 9-, 11-, 13-, 15-, 17-, 19-, 21m depths. A two liter plastic Ruttner sampler was
used.
Analitycal
techniques
pH, salinity, redox potential and oxygen concentration were measured on
line using WTW equipment. Cations concentration was measured by atomic
absorption/emission spectrometry (Spectrometer Varian type AAS 10) with
exception of iron II, which was analysed photometrically using the
1,10-phenanthroline method. Anion analysis were made by ion chromatography or
titration methods.
Modelling
The hydrogeochemical simulator PHREEQC (Parkhurst and Apello, 1999) was
used to calculate saturation indexes using constants included in the
program.
4. Results
Composition of lake water
pH value has been changing in a different way in mikso and monimolimnion
(fig. 2). In 1981y pH was nearly the same at all depths. The small increase of
its value, by 0,2 unit, was noticed between 18 and 20m. From 1988y pH values
have differed significantly from the upper and lower strata. Miksolimnion pH
has been staying on the same level as at the beginning, but monimolimnion pH
has been increasing systematically and reached in 2008 values about
4,8-4,9. Also chemocline has changed
its location over time, from about 9-11 m initially to between 7 and 8 m below
water level currently.
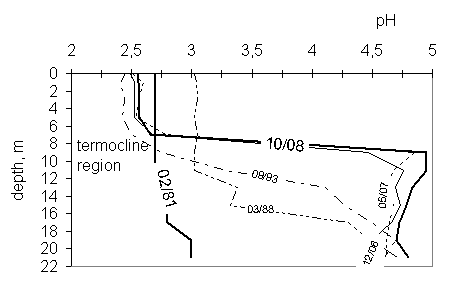
Fig.
2 pH
profiles in the years 1988-2008
The iron concentration in the miksolimnion has been almost constant with
depth, and there was also no tendency of change with time. The concentration of
iron in monimolimnion has been increasing with depth over research period. The
changes of iron concentration have two profiles. The first one (1981 and 1988y)
presents continuously increase of iron amount from the surface to the bottom.
In the second profile, there is a sharp boundary, about an order of magnitude,
between concentration in the upper and lower part of water body. Two zones of
iron concentration in the monimolimnion can be distinguished:
1. in the years
1981-88 concentration varied from about 100 to more than 800 mg/dm3;
2. since 1993y - from
about 100 to more than 1500 mg/dm3 (fig. 3).
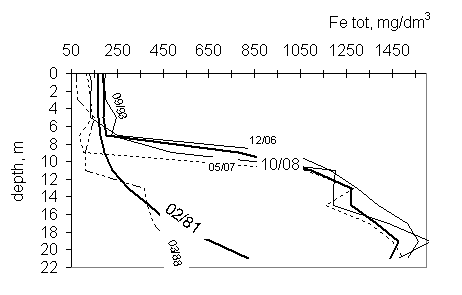
Fig.
3 Total iron profiles in the years 1988-2008
Sulphate concentration in miksolimnion was constant with depth, although
decreased slightly with time (fig. 4). In monimolimnion sulphate content has
been increasing with depth. Since 1993
lower concentration of sulphate in the layer 0-7 m, rapid increase between 7-9
m to the values about 3000 mg/dm3, and then constant increase to a
max concentration about 4300 mg/dm3 has been observed.
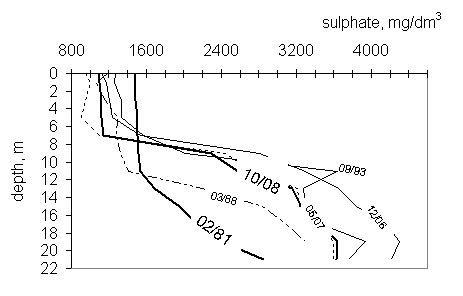
Fig.
4 Sulphate profiles in the years 1988-2008
Two different profiles describe aluminium concentration. In 1981y
aluminium concentration was the highest over research time and increased with
depth. In the years that follows, the concentration of aluminium decreased with
time and depth from about 35 to less than 10 mg/dm3 (fig. 5).
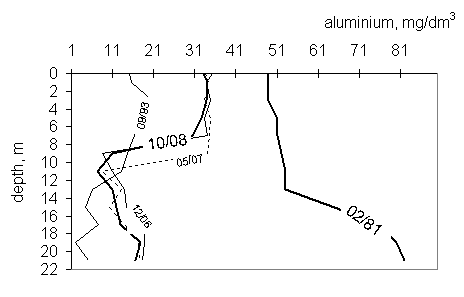
Fig.
5 Aluminium profiles in the years 1988-2008
In 1981y potassium concentration was low and varied from 3 in
miksolimnion to 7 mg/dm3 close to the bottom (fig. 6). The potassium
amount in miksolimnion hasn’t changed significantly with time. The maximum
concentration, 5.8 mg/dm3, was stated in 2008y. In monimolimnion
potassium concentration has been increasing gradually and exceed 30 mg/dm3
in 2008y.
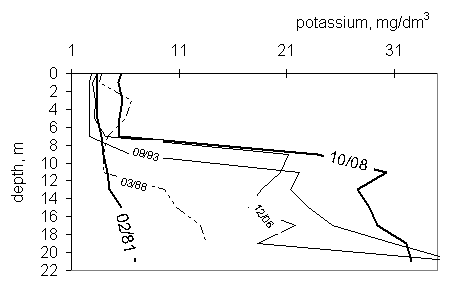
Fig.
6 Potassium profiles in the years 1988-2008
Equilibrium with
solids
Among the secondary minerals, which play the most important role in acid
mine water chemistry the following ferrous salts are mentioned: goethite,
jarosite K and schwertmannit (Evangelou 1985, Stumm, Morgan 1981, Lenk,
Wisotzky 2007). Although all of them have positive SI values in the lake under
research, due to potassium stratification, it seems, that the most important
role in ferrous and sulphate transport from miksolimnion to monimolimnion
played jarosite K precipitation (table 2).
Table
2
SI for chosen solids in 1981 and 2006y
|
Solid name |
formula |
data |
log IS with depth, m below water level |
|||||||||||
|
0 |
1 |
3 |
5 |
7 |
9 |
11 |
13 |
15 |
17 |
19 |
21 |
|||
|
|
Fe(OH)3a |
1981 2007 |
-1,15 -1,32 |
-1,15 -1,34 |
-1,16 -1,29 |
-1,17 -1,11 |
-1,17 -1,18 |
-0,76 *) |
-0,72 |
-0,72 |
-0,69 |
-0,67 |
-0,64 |
-0,64 |
|
goethite |
FeOOH |
1981 2007 |
4,50 4,30 |
4,50 4,18 |
4,50 3,99 |
4,40 4,11 |
3,97 4,01 |
3,39 |
3,39 |
3,67 |
3,17 |
2,85 |
2,59 |
3,16 |
|
jarosite K |
KFe3(SO4)2(OH)6 |
1981 2007 |
2,81 2,83 |
2,66 2,61 |
2,61 2,28 |
2,61 2,57 |
1,7 2,44 |
3,39 |
3,67 |
0,75 |
-0,64 |
-1,44 |
-2,14 |
-0,97 |
|
alunite |
KAl3(SO4)2(OH)6 |
2007 |
|
|
|
|
|
|
3,33 |
3,92 |
2,06 |
2,68 |
5,36 |
6,29 |
|
gibsite |
Al(OH)3 |
2007 |
|
|
|
|
|
|
-0,82 |
-1,15 |
-1,59 |
-1,31 |
-0,05 |
0,43 |
*) lack of value
means very low level
Although at the beginning, the SI for ferrous salts were also positive
in monimolimnion, the amount of ferrous salt was too small, to affect on water
properties. Iron and sulphate have been cumulated in deeper part of water body,
and the reduction of ferrous to ferric influenced on pH values. Over the years
that followed, the pH in monimolimnion reached 4 or more and created the
conditions for precipitation of alum compounds, such as alunite or
gibbsite. SI for this minerals prior to
monimolimnion pH increase so low, that it excluded the possibility of
precipitation.
5. Discussion of
the results
Based on the results presented above, its is difficult unambiguously
describe the origin of meromixis of the lake 54. The main factor it seems to be
enrichment of iron and sulfate due to
the transport from mixolimnion to monimolimnion of secondary minerals. It is
proved, that the chemistry of lake (especially upper layer – monimolimnion) has
been changed due to meromixis and secondary caused its stabilization. Two periods of lake chemical development can
be distinguished:
1. “the iron period” – till 1988y, with intensive processes of ferrous
precipitation in miksolimnion (termocline was then located between 9 and 11m
below water level).
Sedimentation of jarosite K
and may be also goethite, caused transport of ferrous, potassium and sulphate
to the deeper part of water body. On the boundary oxic and anoxic zones
dissolution of minerals took place and caused liberation of ions. It is known
that dissolution of jarosite is incongruent process, but the mechanism of it is
has been not sufficiently described. We can although suppose, that the main
reason of the pH change in monimolimnion was ferrous reduction. This processes
affected on pH values by consuming protons, but didn’t reduce acidity of water
(Schoepke 2001). The potassium stratification confirms the main role of
jarosite K precipitation in miksolimnion.
2. “the iron-alum period” – from 1988 up to now, with two buffering
zones, based on ferrous precipitation in miksolimnion and alum in monimolimnion.
The chemistry of miksolimnion was
similar to that for “iron period”. Chemical composition of miksolimnion didn’t
show any significant changes and was similar to initial composition. The only
difference is alum concentration, which decreased by about 20 mg/dm3,
which means almost 50% of the initial value. Due to the fact, that
monimolimnion acted as a sink for ferrous precipitates, the amounts of
dissolution products has been gradually increasing. The concentration of almost
all (excluding alum) analyzed ions increased significantly. The pH values
increased from about 3 to 4,5-4,9. It created the possibility of alum salts
precipitation, which process lowered alum concentration in monimolimnion and in
a lesser extent, affected on sulphate and potassium concentrations.
6.
Conclusions
1.Meromixis
influenced greatly on the composition of water body in the reservoir 54.
2.Intensive
processes of ferrous salts precipitation in oxic zone and dissolution of the
precipitations with ferrous reduction in monimolimnion, can cause the increase
of pH value of monimolimnion.
3.The increase of
pH the monimolimnion created alum-buffering zone.
4.Monimolimnion
acted as a sink for the products of ferrous salts dissolution, which affected
on water density and stabilized meromixis.
8.
References
Boehrer B, Schulze
M., 2000 On the relevance of meromixis in
mine pit lakes. IMWA/02000-/pp 200-213.
Jędrczak A., Skład chemiczny wód pojezierza antropogenicznego w Łuku Mużakowskim. Wyd. WSInż. w Zielonej Górze, Seria Monografie1992;, Nr 5, ss 135 (in polish)
Solski A., Jędrczak A.,; Meromixis in acidotrophic reservoirs of the „anthropogenic lake district”.
Pol. Arch. Hydrob., 1991a No 38/3/4, 327-346
Solski A., Jędrczak A., Ionic composition of waters of the „anthropogenic lake district”.
Pol. Arch. Hydrob., 1990;No 37/3, 361-382
Solski A., Jędrczak A.,; Forms of iron in waters of acidotrophic- meromictic reservoirs of the „anthropogenic lake district”. Pol. Arch. Hydrob., 1991b No 38/3/4, 315-326.
Jędrczak
A., JachimkoB, Najbar N.: Zmianyfizyczno-chemicznych
cech największego zbiornika na pojezierzu antropogenicznym w okresie
kilkunastu lat.. Zeszyty Naukowe Politechniki Zielonogórskiej
1998(Inżynieria Środowiska 7) nr 116, p.5-18 (in polish)
Parkhurst
D., Appelo C.A.J.: User’s guide to
PHREEQC (Version2)- a computer program for speciacion, batch-reaction,
one-dimensional transport, and inverse geochemical
calculations. Denver, 1999
Evangelou, V.P. Pyrite oxidation and its control; 1995, New York
Stumm, W., Morgan, J.J. Aquatic chemistry – an introduction
emphasing chemical equilibria in natural waters, 2nd ed.; 1981 New York
Lenk S., Wisotzky F.: Chemische Beschaffenheit und mmodellierte
Genese von Grundwassern in Braunkohlenabraumkippen des Tagebaues Inden
Grundwasser (2007) 12:301-313
Schöpke, R.; Koch, R.; Querfelli I.;
Striemann M.; Preuß V.; Regel R.;. Anwendung
des Neutralisationspotentials bei der Bilanzierung von Saeure-Base-Reaktionen
im Umfeld des Braunkohlebergbaues. Grundwasser 1/2001 23-29