Chemistry
Nadirov
R.K.1, Aitkulova R.E.2,
Nadirov K.S.2
1Kazakh National University, Almaty, Kazakhstan
2South Kazakhstan State University, Shymkent,
Kazakhstan
THEORETICAL
COMPUTATION OF ELECTRODE POTENTIAL OF ELECTROHEMICAL OXIDATION OF EUPAFOLIN
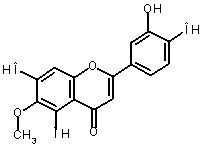
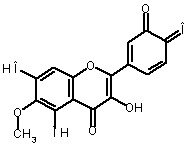
The oxidation of eupafolin (1) in aqueous-acetonitrile solution
involves a two-electron process, which is represented below:
|
-2Í+ -2å (1) |
(1) (2) |
Alternatively, eupafolin can
be converted to its oxidized form (2)
using o-benzoquinone (3) as a
reference molecule according to the following isodesmic reaction [1]:
|
(1) |
+ |
(3) |
(2) + |
(2) (4) |
The difference between the
electrode potential of two species can be obtained from the change in Gibbs free energy of reaction (2) [2]:
![]() (3)
(3)
where n is number of electrons
transferred (n = 2 in this case) and F is the Faraday constant.. In order to
obtain standard electrode potential of eupafolin, the standard
change of Gibbs free energy of reaction (2), ΔG0, is
required along with the experimental value of electrode potential of the
reference molecule, ortho benzoquinone [3]. In order to calculate the standard
Gibbs energy of reaction (2), ΔG0, one should calculate the
standard Gibbs energy of each component, ![]() , in reaction (2) and
this is possible by calculation of the gas-phase energy of each component,
, in reaction (2) and
this is possible by calculation of the gas-phase energy of each component, ![]() , together with the solvation energy of the component,
, together with the solvation energy of the component, ![]() [2]:
[2]:
![]() (4)
(4)
![]() (5)
(5)
Thus, Gibbs energy of each
molecule in the gas phase is necessary for the calculation of electrode
potential of eupafolin.
According with [2], in the
present work, the gas phase contribution to the Gibbs energy, ![]() , was determined from DFT calculations [3].
, was determined from DFT calculations [3].
The entropies have been used to convert the internal energies to the
Gibbs energies at 298 K. We have used polarizable continuum model (PCM) of
solvation [5] in order to calculate solvation energies, ![]() . HYPERCHEM (7.0) software have been employed for all DFT
calculations.
. HYPERCHEM (7.0) software have been employed for all DFT
calculations.
Table shows the calculated Gibbs energy of
molecules for both reduced and oxidized forms in the gas phase. Solvation
energies are also computed in order to convert gas-phase energies to energies
in solution phase. The total Gibbs free energy of each component in the
presence of solvent are also included in Table 1.
Using these values together
with the standard Gibbs free energy of pyrocatechol (4) which are presented in Table,
and employing Eqs. (3) and (5), the standard electrode potential of eupafolin
is calculated to be 0.724 V.
Table –
Heat of formation, entropies and Gibbs free energies for oxidized (2) and reduced (1) forms of eupafolin and also o-benzoquinone (3) and pyrocatechol (4)
|
|
Heat of formation, kj/mol (gas fase) |
Entropies, kj/mol•K (gas phase) |
Gibbs free energies, kj/mol |
|
|
Gas phase |
Aqueus phase |
|||
|
(1) |
-851.473 |
0.336 |
-951.601 |
-951.719 |
|
(2) |
-665.494 |
0.317 |
-759.960 |
-760.020 |
|
(3) |
-95.187 |
0.117 |
-130.053 |
-130.362 |
|
(4) |
-263.223 |
0.098 |
-292.427 |
-292.517 |
All energies are at 298 K
References
1.
B.A. Bohm, Introduction to Flavonoids, Harwood Academic Publishers,
Singapore, 1998 (Chapter 2).
2.
Hamid R. Zare, Mansoor Namazian, Navid Nasirizadeh, J. Electroanal. Chem
584 (2005) 77.
3.
M. Namazian, P. Norouzi, J. Electroanal. Chem. 573 (2004) 49.
4.
A.D. Becke, J. Chem. Phys. 98
(1993) 5648.
5.
S. Miertus, E. Scrocco, J. Tomasi, Chem. Phys. 55 (1981) 117.