Prof. S. Shestakov1, prof. O. Krasulya1,
prof. E. Smeshek2
1 Moscow State University of Technology and
Management, Russia
2 Polessky State University, Belarus
Sonochemistry of food - area
high-energy chemistry which actively is researched now in Russia and Belarus
Sonochemistry as a field of science is relate to the
subject of physical chemistry and is a part of high-energy chemistry.
Sonochemical method allows to have a direct impact on the reagents in
endothermic liquid-phase reactions and do not require heating of the solution
containing these reagents. The main factor of sonochemical reactions is the
giant pulses of fluid pressure from pulsating of cavitation bubbles - acoustic
cavitation. Bubbles in the compression phase is reduced in diameter to nanometer
dimensions, the gas-vapor mixture inside them are heated and turns into a
plasma, what is accompanied by the emission of photons - the sonoluminescence.
These rays can reach the energy of ultraviolet radiation. There are even
attempts to produce in cavitation bubbles filled with pairs of deuterated
acetone, a inertial thermonuclear fusion [1].
Reactions of hydration , in contrast to the hydrolysis
reactions is not accompanied by the dissociations of the molecules, but the
modern chemistry of tends to attribute
them to the chemical reactions, because, having polarity molecules the water
itself causes dissociation into ions of dissolved substances. Using this
approach, which limiting the food sonochemistry by reactions aimed at the
dipole-dipole and ion-dipole interactions in aqueous solutions, in Russia and
Belarus in recent years studies are in this area. Developed unique research
methods of sonochemical processes and devices, as well as technologies for food
and drug sonochemistry and the sonochemical reactors for their implementation.
In 2010, was successfully completed the state sanitary-epidemiological
inspection and certification of cavitation reactors special series to implement
sonochemical technologies in the food industry.

Setting
sonochemical processing of solutions salt used in products from minced meat.
One of the world's
leading food sonochemistry scientists – Dr. M. Ashokkumar, Professor,
University of Melbourne in 2011 visited his Russian colleagues-researchers. He
estimated the undertaken in Russia the approach to food sonochemistry, praised
of their research and myself took part in them [2,3].
Earlier sonochemical is thought only the
processes that occur in the gas phase inside the cavitation bubbles [4]. One of
the reactions of water, which not accompanied the dissociation of water
molecules – the destruction by pulses pressure from the ripple bubbles by
molecular structure of water which is formed by hydrogen bonds. The presence of
this structure, reminiscent of the structure of ice, even at room temperature
was once again confirmed by studies at Leiden University [5] and is associated to the polarity of the water
molecules. Reaction which break this structure by mass reagent (water) immeasurably
superior of reactions the pyrolysis in vapor phase of bubbles.
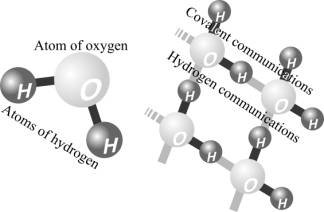
The water molecule and molecular structure of water
which is formed by hydrogen bonds.
As a result sonochemical reaction dehydration,
the water for a while loses structure and becomes thermodynamically
nonequilibrium state. In the Institute of Chemical Physics of Russian Academy
of Sciences by measuring proton magnetic relaxation in distilled water which
was subjected to sonochemical processing in the cavitation reactor, was
established how long this water returned to equilibrium. There established the
presence in the water after the treatment phases with different molecular
mobility, the separate existence of which there is approximately 2-3 hours.
During this period, energy of cavitation the
received by water is transformed into heat of hydration of water molecules
themselves, that is, into heat from recovery of hydrogen bonds in an amount
corresponding to the thermodynamic equilibrium.
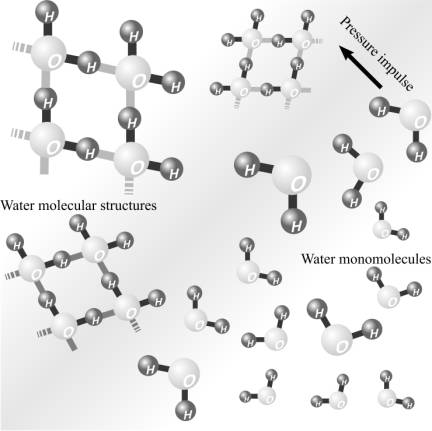
Scheme of destroying the molecular structure of water by cavitation.
The strong increase in the
viscosity of water as the temperature decreases, a paradoxical decrease in
density when cooled below +4°C, and high in comparison with non-polar
liquids, the surface tension is explained with phenomenon the self-hydration.
Unrelated molecules of water may to hydrate of biomacromolecules, creating
dense hydration shells around them. Hydration is the process of binding water
chemicals - chemical reaction, which produces a new substance. Chemistry of
biopolymers known that, for example, a protein with a molecular structure of
amino acids which have carboxyl –COOH, hydroxyl –OH and amine –NH2
polar groups, is capable as a result of hydration attach up to 40% water by
weight. [6] According to the teachings of Acad. V. Vernadsky, the water which
is connected in hydration is an integral part of the protein. It naturally
increases mass of protein, because actions the mechanisms similar to those that
occur during its synthesis and almost equally well how strong peptide bonds.
Food raw materials in today for the most part kept in the dried or frozen, that
is, has the loss of natural moisture or losing a bond with her. Therefore,
finding the ability to manage the hydration of biopolymers solves a huge
problem - reducing the number or excludes from food, nonfood substances, which
are traditionally used to artificially increase the binding of water and in
this way their mass.
Hydration process management
has become one of the main directions of development of food and drug
sonochemistry in Russia [3]. Hydration capacity of water in relation to other
substances depends on content in water of unstructured phase. When a source of
energy of disintegration of the the hydrogen bonds is disappears, they again
begin to recover in the amount corresponding to the thermodynamic equilibrium,
returning the absorbed energy as heat self-hydrations. The hydrogen bonds
disintegration by impulse of pressure cavitation occurs as is shown in figure:
![]()
![]()
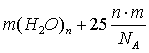 kJ, (1)
kJ, (1)
where:
m – the number of molecular water associates in the reaction; n –
the number of water molecules is forming a stable associates; NA –
Avogadro's number.
Since the heat of vaporization
of water is equal to 44 kJ/mol, then she may be to devoid of structure
by heating. But if the goal is to increase the degree of hydration of
biopolymers, which are themselves unstable to thermal denaturation, thermal
fracture mechanism of hydrogen bonds is not acceptable. There are many known
ways of destruction of the structure of water without her heating. These
include all methods of mechanical action, such as processing in a colloid mill
or in a disintegrator of rotary type, and sometimes transfer the energy using
the polarity of water molecules [7]. A researcher working with the water is
prepared by the last way for kneading dough on baking, found that thickness of
the hydration shells of protein molecules is reduced and formed a more flexible
structure of the protein. This supports the hypothesis of the structuring of
protein by means hydration [8]:
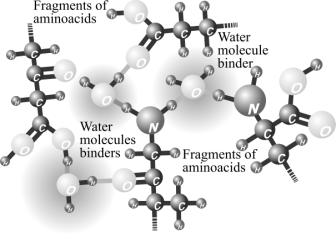
The structuring of protein by
means hydration
These techniques improve the
solvent power of water is doing by means changing her energy state at the
expense of energy transformation different nature, temporarily deducing it from
thermodynamic equilibrium. The advantage of sonochemical effect in is that the
original chemical composition of the water does not matter. On the contrary,
the water may contain dissolved in any amount or suspended solids and they will
not be lost in the process of disintegration, as in the case of membrane
cleaning and do not to form undesirable chemical compounds as at electrolysis.
The latter is particularly important because for foods the content of mineral
substances and the content of useful trace elements is controlled by dissolving
them in incoming water. The cavitation in water is accelerate dissolution, ions
of dissolved substances get the dense hydration shells, which reduces their
ability to participate in unwanted chemical reactions. This is another advantage of sonochemistry.
Known a method of processing
grain aqueous solution of propionic acid before placing it into storage [9] and
the method of wetting grain with water (his conditioning) at production from it
flour [10]. In these methods, the liquid is exposed to ultrasound. Acoustic
processing power is not set explicitly, but in the second case is required to
form hydroxyl ions and synthesis of hydrogen peroxide which is known to be
implemented in two ways:
OH + OH → H2O2
(2)
O + HOH → H2O2, (3)
where one of its part formed from of the synthesized hydroxyl ions, in
the other is involved dissolved in the water oxygen [11]. Therefore in [10] is
provided preliminary oxygen saturation of water by means forced aeration. The
remaining hydroxyl ions in the water further destroy the structure of the grain
due to the effect of ion cracking. A hydrogen peroxide has detrimental effect
on the microflora and decomposed by enzymes of grain. Decomposition has
explosive character, which also destroys the endosperm of kernels. But this
method of preparation of water for hydration food stuff which include fats is
not suitable, because they can oxidize, leading to a deterioration of taste and
reduce the shelf life of products prepared from hydrated that way biomass. In
Russia and Europe was patented a sonochemical method of hydration of proteins
meat by water which processed in cavitation reactor at the amplitude of the
sound pressure in the 2 ... 23 times higher than the hydrostatic pressure in
him [12]. This method is not widely adopted due to short shelf life of foods,
such as sausages made with its use, even though it saves raw materials [13].
In Russia created the method
sonochemical processing of brine [14] and sonochemical method of processing the
water and water solutions to hydration biomass [15]. In they hydrogen peroxide
is not synthesized in significant quantities, because the amplitude of the
ultrasonic reactor pressure does not exceed twice the value of the hydrostatic
pressure in the liquid. But the decrease of the pressure amplitude of
ultrasound which causes cavitation and whose square of which is proportional
the acoustic power of the process, has led to a drastic decrease of performance
of the sonochemical processing. From the general physics we know that necessary
for any action energy is equal to the product of the power of the impact on its
duration. Therefore, such methods in the production of, for example, meat
products have been used only in catering [13], where produced small quantities
of products.
But there is a way of hydration of biopolymers by sonochemical processed
water or solutions on its basis [16]. In it sonochemical processing carry out
in the cavitational reactor with an average amplitude of sound pressure of the
elastic wave exceeding hydrostatic pressure not less than in 5,5 times. In the
description of this method examples of its implementation are given. In the
first example at hydration of proteins of a gluten of grain of wheat is
received the increase in weight of a crude gluten and reduction of the general
microflora. It is known that the adipose component of the grain is in its
embryo, which prior to processing of grain into flour is separated from the
grains. Therefore, the method has shown here a positive result. In the second
example sonochemical treatment of an aqueous suspension of mustard seeds to
extract from it the substances used in the manufacture of mayonnaise,
microbiological purity of the suspension medium and the content of organic
acids in it increased. In the following example as a result of water processing
for preparation of a brine at production semi-finished products from chopped
meat the content of microflora in forcemeat decreased, fat – is increased. It
is known that the analysis of the content of fat in foodstuff and semi-finished
products is carried out methods of extraction by Soxhlet and Randall. Therefore
it is possible to assume that the paradoxical increase in the content of fat in
meat is connected with the increased extractability of products of its
oxidation which formed at hydration by processed water. The given examples of
implementation [16] confirm that oxidizers in water nevertheless are formed in
sufficient quantities for oxidation of fats in a hydrated biomass.
Problem of the last researches conducted
by us is search of a way of decrease in the content in the processing water,
including, which being the environment of solution or disperse system,
oxidizers like hydrogen peroxide without decrease in acoustic power of the
sonochemical processing, and without to reduction its productivity.
In [11] are given experimental data about
H2O2 exit depending on the spent energy at synthesis by
ultrasonic fluctuations. Is noted, what hydrogen peroxide exit strongly depends
on a chemical composition of water, in particular, from the content in it the
dissolved oxygen of air. Later was a published result of researches about an
exit the hydrogen peroxide in the two-factorial experiment [8]. By means of it
it is established that this exit is optimized by function of two variables.
Existence of a local maximum is explained with by heating of water from
internal friction, decrease in the contents in it of oxygen and thermal
decomposition at increase H2O2 in time of processing and
acoustic power high over optimum values. It is known also that coincidence of
collapse of cavitational bubbles practically any primary diameter in water from
the end of the period of an acoustic wave which causing cavitation is comes
with amplitude of pressure approximately equal to five hydrostatic pressure
[13]. It is a so-called mode of sinperiodic cavitation. At increase above this
value the potential energy responsible for the size of a cavitational erosion
and a sonoluminescence both in singlebuibble and in multibubblle cavitation
remains to constant, and the kinetic changes only. Thus, the mode of
sinperiodic cavitation is most energetically favorable. But, as it was shown
above, usage [16] in the food industry is hinder the education in hydrogen
peroxide water in significant quantities. If to consider that the exit of
hydrogen peroxide depends on the content in water of the dissolved oxygen of
air, there was a hypothesis that is possible to lower it, previously having
subjected water or water solution of deaeration.
In [17] is described the fact of an invariance of
permanganat oxidability of solutions of free organic acids, which is subjected
sonochemical processing with an amplitude of acoustic pressure no more than 2 atm.
Influence of processing on hydration ability of water was estimated there by
efficiency of dissolution of tableted NaCl. Therefore experimental check of
correctness of this hypothesis it was carried out as follows.
Expenses of energy necessary on sonochemical water
processing are established in [15]. At the room temperature they make about 2 kW×h/m3.
In reactor described in [17] is installed the magnetostriction converter with
an electric power of 630 W, its absolute productivity makes 0,16 m3/h.
Productivity of the reactor of the Oil Tech Production OY company
(Tallinn) in [18], used in experiments, with piezoelectric converters is equal
1,08 m3/h, that is amplitude of sound pressure here is
2,6 times more and is equal to amplitude of sinperiodic cavitation. Considering
it, prepared three identical samples of solution of 0,4 mg/l tannin
which strongly predisposed to oxidation by
hydrogen peroxide. The permanganat oxidability of solution measured by a
method of Kubel, made 5,2 mgO2/l. The first sample
considered control and within 3 min in experimental installation
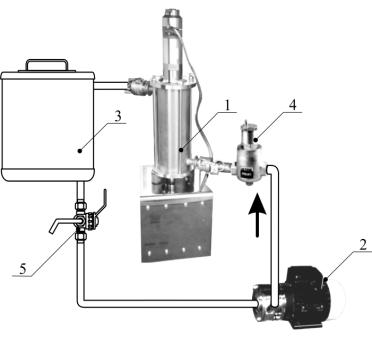
Experimental
installation with the cavitational reactor of the company Oil Tech
Production OY:
1
– reactor; 2 – pump; 3 – to a receiver;
4
– deaerator Caleffi 551; 5 – three-running crane.
recirculated it by
means of the pump via the reactor which was disconnected, receiver and turning
on deaerator after the pump. Method [16] was doing out on the second sample
with recirculating of solution within 1 min. The solution by means of the pump
passed via the switched-on sonochemical reactor, a receiver and at the
deaerator. Hypothesis checked on the third sample is processing it as follows:
within 2 min solution recirculated by means of the pump via the switched-off
sonochemical reactor, a receiver and deaerator, then for 1 min turned on the reactor. In all three cases
after the expiration of time of processing the pump disconnected, the crane to
solution plum and measured on ISO 8467 permanganate oxidability. In total five
series of experiences were doing. Results are shown in the table in the form of
average values with ranges of mean square deviations.
Table
|
parameter |
Unit |
value |
||
|
Sample 1 |
Sample 2 |
Sample 3 |
||
|
Permanganate oxidability |
mgÎ2/l |
5,12±0,05 |
2,31±0,07 |
5,09±0,05 |
From the table it is visible that permanganate
oxidability of samples 1 and 3 is almost identical whereas at a sample 2 it is
lower. It means that the part of tannin was oxidized by the hydrogen peroxide
which formed in the course of sonochemical processing. Further compared results
of dissolution of the tablets NaCl during identical time by immersion them in
the processed and non processed water. Results for sample 3 is similar to a
photo from [17]. It is visible that the difference of reduction of volume of
tablets dissolved in usual and to the sonochemical processed water is almost
identical. But in a sinperiodic mode it was required to time for sonochemical
processing much less.
|
|
+
Tableted NaCl after
exposure to normal water a
(a) and water, which
subjected sonochemical processing b (b) in accordance with [17]
(top) and with [16], but with deaeration (bottom).
The
experiments have confirmed the validity of the hypothesis, as the yield of
hydrogen peroxide really depends on the content in the water of dissolved
oxygen. This allows you to use in the food sonochemistry most energetically
favorable mode the sinperiodic cavitation [13] subjecting the water or an
aqueous solution of forced deaeration. At the same time hydration and solvent
power of water can not fall off and time for sonochemical processing required
less than traditionally accepted in food sonochemistry. That is use in the food
industry the sonochemistry will be absolutely safe, if the take action which
prevent the formation of hydrogen peroxide in water way remove from the
solution the atmospheric oxygen.
References:
1. Taleyarkhan R. et al. Evidence for Nuclear Emissions
During Acoustic Cavitation. Science, V.295, 2002, 1868-1873
2. Ashokkumar M., Rink R.,
Shestakov S. Hydrodynamic cavitation – an alternative to ultrasonic food
processing. Electronic Journal “Technical Acoustics”, http://www.ejta.org,
2011, 9
3. Ashokkumar M. et al. A New Look at Cavitation
and the Applications of Its Liquid-Phase Effects in the Processing of Food and
Fuel. Applied Physics Research, Vol. 4, No. 1, 2012, 19-29
4. Margulis M. Basics
sonochemistry. Chemical reactions in the acoustic field. (Moscow: High School, 1984) (in Russian)
5. Jinesh K. B.,
Frenken J. W. M. Experimental evidence for ice formation at room temperature.
Physical Review Letters, 101, 2008, 036101.
6. Kuntz I.D. The physical properties of water
bound to biomacromolecules, in R.B. Duckworth (Ed.) Water relations of foods
(London: Academic Press, 1975)
7. Korchagin V.I. et al. Use in
baking temporarily activated
water. Baking in Russia, 5, 2000, 16-18 (in Russian)
8. Shestakov S.D. Basics of cavitational disintegration.
(Moscow: EVA press, 2001) (in Russian)
9. Patent RU 2122311,
1998
10. Patent RU 2171568, 2001
11. Flynn G. Physics of
acoustic cavitation in liquids,
in W. Mason (Ed.) Methods and devices
of ultrasonic researches (V.1, Part B, New York,
London: Academic Press, 1964)
12. Patent EP 1609368,
2007
13. Shestakov S. Food
sonochemistry: concept, theoretical aspects and practical applications
(Saarbrücken: LAMBERT Academic Publishing, 2012)
14. Patent RU 2402909,
2010
15. Patent RU 2444201,
2012
16. Patent application
WO/2007/111524, 2007
17. Krasulya Î.N., Shlenskaya T.V., Shestakov S.D. Experience using
sonotechnology in the food industry, Proc. XXII Session of Russian Acoustic
Society, Vol. 2, Moscow: GEOS, 2010, 68-74
18. Rink R., Shestakov
S., Babak V. The Sonochemical Reactors with Symmetric oscillatory Systems of
the Acoustic Cells International Journal of Research and Reviews in Applied
Sciences, Vol. 12, 3,
2012, 391-396