Kutsenko O.K., Trusova V.M., Gorbenko G.P.
V.N. Karazin Kharkiv National University, 4 Svobody Sq.,
Kharkiv, 61077, Ukraine
Cholesterol effect on hemoglobin-lipid
interactions
Cholesterol
(Chol) effect on hemoglobin (Hb) binding to lipid bilayer is a problem
attracting considerable attention. In particular, specific sterol location in
phospholipid bilayers stabilizes Hb structure, prevents protein oxidation,
dissociation and loss of its physiological activity [1]. Furthermore, it was recently found that cholesterol, associated with phospholipids, is
capable of forming complexes with Hb in human erythrocytes. Although these
complexes exert influence on the cell antioxidant system, they do not directly
interact with reactive oxygen species. The role of Hb-Chol complexes is thought
to involve the shifts of correlations in the erythrocyte defense system [2,3].
|
|
|
|
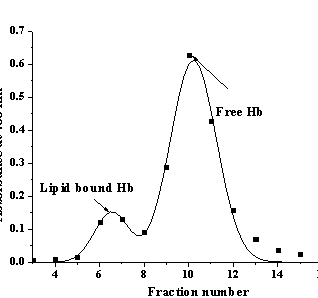
Fig. 1. Elution profile of Hb-liposome mixture |
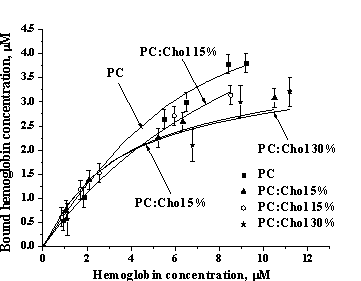
Fig. 2. Hb-lipid adsorption isotherms. Lipid concentration 1 mM. |
The present work was focused
on examination of Hb binding to the model membranes composed of zwitterionic
lipid phosphatidylcholine (PC) and its mixtures with cholesterol (Chol). Hb
adsorption onto lipid bilayer was studied using the size exclusion
chromatography on the gel Toyorearl HW-60F. Fig. 1 represents typical elution
profile of Hb-lipid mixtures, in which the first peak corresponds to the
protein-liposome complexes and the second peak – to the protein free in
solution. A set of such profiles obtained for different Hb concentrations yielded
adsorption isotherms presented in Fig. 2. Experimental binding curves were then
quantitatively analyzed in terms of the lattice model of large ligand
adsorption to membranes allowing for the possibility of protein insertion into
bilayer interior (Eqs. 1-2).
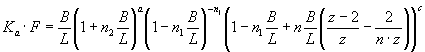 ,
Eq. 1
,
Eq. 1
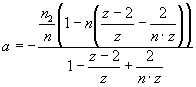 ,
, 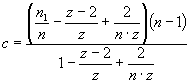 ,
, ![]() , Eq. 2
, Eq. 2
where Ka – association constant, F – concentration of Hb free in
solution, B – concentration of lipid-bound
Hb, L – lipid concentration, n1, n2 – the
number of lipid molecules constituting the binding sites for surface-bound and
bilayer-inserted Hb, respectively, n=n1+n2 – total number
of lipids per bound protein, z = 6 – coordination
number for hexagonal lattice, ΔG
– free energy change, R – universal gas constant, T – temperature.
This
thermodynamic model takes into account specific features of protein-lipid complexation,
such as large size of the protein, ability of Hb to form contacts with several lipids,
and steric area-excluding interactions between absorbed protein molecules. Analysis
of the data presented in Table 1 suggests that Chol may affect both Hb-lipid
binding energy and stoichiometry.
Table 1
Thermodynamic parameters of Hb-lipid binding
|
System |
n |
Ka, M-1 |
ΔG, kJ/mol |
|
PC |
17±2,5 |
(1,5±0,2)×104 |
-23,8±3,6 |
|
PC:Chol (5 mol%
Chol) |
33±5,0 |
(1,9±0,3)×104 |
-24,4±3,7 |
|
PC:Chol (15 mol%
Chol) |
20±3,0 |
(3,8±0,6)×103 |
-20,4±3,1 |
|
PC:Chol (30 mol%
Chol) |
33±5,0 |
(2,5±0,4)×104 |
-25,1±3,7 |
Several lines of evidence
indicate that sterol effect on physicochemical properties of a lipid bilayer
may involve: i) increased phospholipid headgroup
separation, ii) increased freedom of
motion of phosphorylcholine moiety, iii)
ordering of lipid hydrocarbon chains (condensing effect). These bilayer
perturbations may be coupled with Chol-mediated Hb conformational changes,
protein oxidation and dissociation of heme-globin complex. Such a behavior may
manifest itself in the increased constant of Hb binding to model membranes
containing 30 mol% of Chol as compared to neat PC bilayer.
Non-monotonous
dependence of Ka changes
on Chol content may be rationalized in terms of the lattice model of lipid
lateral distribution. Briefly, this model implies the variations in membrane
free volume in two-component lipid bilayers stemming from the difference in
cross sectional areas between the guest (Chol) and matrix (PC) lipids. The
extent of membrane free volume fluctuations varies periodically with mole
fraction of the guest lipid. It may be assumed that these free volume
fluctuations underlie the nonlinear dependence of binding parameters on Chol
concentration.
This
work was supported by the Grant number 4534 from the Science and Technology
Center in Ukraine.
1.
Szebeni J., Hauser H., Eskelson C.D., Watson R.R., Winterhalter
K.H. Interaction of hemoglobin derivatives with liposomes. Membrane cholesterol
protects against the changes of hemoglobin // Biochem. J. – 1988. – Vol. 27. –
P. 6425-6434.
2.
Nicolic M., Vranic D., Spiric A., Batas V., Nicolic-Kokic
A., Radetic P., Turubatovic L., Blagojevic D.P., Jones D.R., Nicetic V., Spasic
M.B. Could
cholesterol bound to haemoglobin be a missing link for the occasional inverse
relationship between superoxide dismutase and glutathione peroxidase
activities? // Biochem. Biophys. Res. Commun. – 2006. – Vol. 348. – P. 265-270.
3.
Nicolic M., Nicolic-Kokic A., Stanic D., Blagojevic
D.P., Vranic D., Jones D.R., Niketic N., Spasic M.B. Does cholesterol bound to
haemoglobin affect the anti-oxidant enzyme defence system in human
erythrocytes? // J. Serb. Chem. Soc. – 2007. – Vol. 72. – P. 339-345.